1. Introduction
In December 2019, an outbreak of coronavirus 2019 (SARS-COV-2) was reported in Wuhan, a city in China, which spread to other cities in China and to other countries in the world (1). The outbreak of SARS-COV-2 has been declared a global public health emergency by the World Health Organization (WHO) (2). The virus is transmitted through respiratory droplets or contact, and human-to-human transmission has been reported (3).
Angiotensin-converting enzyme 2 (ACE2) is indicated as a receptor for SARS-CoV-2, which is expressed in human placenta (4). Thus, with the outbreak of SARS-COV-2, the prevention and control of the infection in pregnant women and the potential risk of vertical transmission have become a major concern (5-7).
There is still insufficient information to determine the effect of SARS-COV-2 infection on the fetus. Whether SARS-COV-2 has a maternal vertical transmission is still unclear (8). However, some studies have provided information on the clinical features, pregnancy outcomes, and potential of vertical transmission of SARS-COV-2 infection in pregnant women (5, 7, 9), but more evidence is needed to develop effective preventive strategies (8, 10).
Following the crisis of SARS-COV-2 disease in different parts of the world, this outbreak in Iran was officially approved in February 2020. Guilan province is one of the 31 provinces of Iran and was one of the first provinces with SARS-COV-2 cases. Since studies on neonates with SARS-COV-2 are limited (11), our aim is to introduce a case of neonatal SARS-COV-2 infection in Guilan province of Iran to share our experience with those involved in the care and management of this disease. In addition, we performed a short review of the literature related to this case.
2. Case Presentation
On March 11, 2020, a 36-week pregnant woman visited Pirooz Hospital, a general hospital in Lahijan city of Guilan province, due to fever, cough, and respiratory distress.
The fever and cough had begun a week ago, but the patient had only taken acetaminophen tablets to control fever. She was gravid 1 and had no specific diseases. She had had exposure to a suspected SARS-COV-2 case in her family.
On admission, her vital signs were as follows: body temperature 38.5°C, respiratory rate 27 breaths per minute, pulse rate 125 per minute, and blood pressure 90/60 mmHg. The patient’s O2 saturation (SpO2) was 60% to 70%. Fetal heart rate (FHR) was 80 - 100 bpm, and fetal heart monitoring showed fetal distress. Ultrasound examination showed oligohydramnios, but the exact time of rupture of membranes was not known, and the patient mentioned a sense of discharge one day before admission.
Possible SARS-COV-2 pneumonia was diagnosed based on the patient’s clinical presentations and history of exposure. The treatment plan was given based on the hospital’s protocol for SARS-COV-2. Meropenem (1 g stat, then 1g every 8 hours intravenously), vancomycin (1 gr stat, then 1 g every 12 hours intravenously), hydroxychloroquine (200 mg stat, oral), kaletra (400 mg every 12 hours, oral), and Tamiflu (75 mg every 12 hours, oral) were prescribed.
An emergency cesarean section was performed for the patient because of fetal distress. Meconium staining was shown intraoperatively. A female neonate with weight 2900 g, height 48 cm, and head circumference 33 cm was delivered. Apgar scores at 1 and 5 minutes after birth were 7 and 8, and the neonate was transferred to the neonatal intensive care unit (NICU) because of respiratory distress, and the mother transferred to the intensive care unit (ICU).
Immediately after birth, the newborn was transferred to the NICU with a sterile incubator and placed in an isolated room. There was not any contact between the mother and the infant. The treatment team followed individual protective conditions and infection control principles during patient management.
The result of a pharyngeal swab sample for real-time reverse transcription polymerase chain reaction (rRT-PCR) showed positive SARS-COV-2 for the mother. However, samples for the SARS-COV-2 test were not taken from the amniotic fluid, cord blood, and placenta. Unfortunately, the mother died after 4 days of hospitalization in the ICU.
On arrival to NICU, the newborn had severe respiratory distress with respiratory rate 70 per minute, intercostal and subcostal retractions, and SpO2 82%, so she was intubated. The neonate was connected to a ventilator for respiratory support, and surfactant (2.5 cc/kg) was injected intratracheally, then SpO2 reached 95% with PIP = 30 cmH2O, ventilator rate = 60 and PEEP = 5 cmH2O. A second dose of surfactant (1.25 cc/kg) was injected intratracheally 12 hours later due to high FiO2 requirement, and then PIP and FiO2 were gradually diminished. The neonate was extubated 24 hours after birth and was given respiratory support with nasal continues positive airway pressure (C-PAP).
The neonate was echoed at the first 12 hours of birth. The first echocardiography showed ejection fraction (EF) 40%, mild pulmonary hypertension (PH), and left and right ventricular dilation.
Milrinone with the loading dose of 75 mcg/kg was infused over 60 minutes with the maintenance infusion of 0.5 mcg/kg per minute, immediately after a loading dose of 1 mg/kg furosemide was given every 12 hours and 1 mg /kg spironolactone was administered daily.
On the third day of birth, a second echocardiography showed EF: 35%, PH: 37 mmHg and mild mitral regurgitation (MR), and 10 mcg/kg/min dobutamine and 1 gr/kg intravenous immunoglobulin (IVIG) were given.
On the fourth day of birth, pharyngeal and nasal mucosa specimen were collected for SARS-COV-2 test, which was positive for the neonate.
On the fifth day of birth, the third echocardiography showed EF: 55% and trivial MR. Gradually, the cardiac medications were reduced, and she was separated from nasal C-PAP and received oxygen through the hood, and formula feeding was begun. After 10 days, all the medications were discontinued, and the IV line was withdrawn. Finally, the baby was discharged on March 26, 2020, with improvement in heart and respiratory status and in good general condition.
The neonate laboratory test results are shown in Table 1.
| Test | In Patient | Normal Range |
|---|---|---|
| WBC (× 109/L) | 13.2 | 9 - 30 |
| Hb (g/L) | 12.6 | 12 - 16 |
| PLT (× 109/L) | 218 | 150 - 350 |
| CRP(mg/L) | 0.1 | 0 - 0.8 |
| Ca (mg/dL) | 8.2 | 8 - 11 |
| Na (mmol/L) | 137 | 136 - 145 |
| K(mmol/L) | 3.8 | 3.5 - 5 |
| AST (U/L) | 36 | 0 - 35 |
| ALT (U/L) | 46 | 0 - 35 |
| Urea (mg/dL) | 19 | 10 - 40 |
| Cr (mg/dL) | 1.1 | 0.7 - 1.3 |
| CPK ( U/L) | 1666 | 195 - 700 |
| LDH (U/L) | 2032 | 160 - 450 |
Troponin was negative. Blood gas analysis of the neonate showed (first day of birth): pH: 7.08 (normal range: 7.30 - 7.35), PCO2 (mmHg): 50 (normal range: 35 - 45), HCO3 (mEq/L): 9.5 (normal range: 22 - 25), BE/BD (mmol/L): -1.7 (normal range: ±3), SpO2: 82%, blood group: O+, and blood culture: no growth.
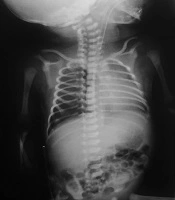
The first chest X-ray (CXR) after intubation: Except mild hyperinflation and cardiomegaly, no other significant finding was visible (Figure 1).
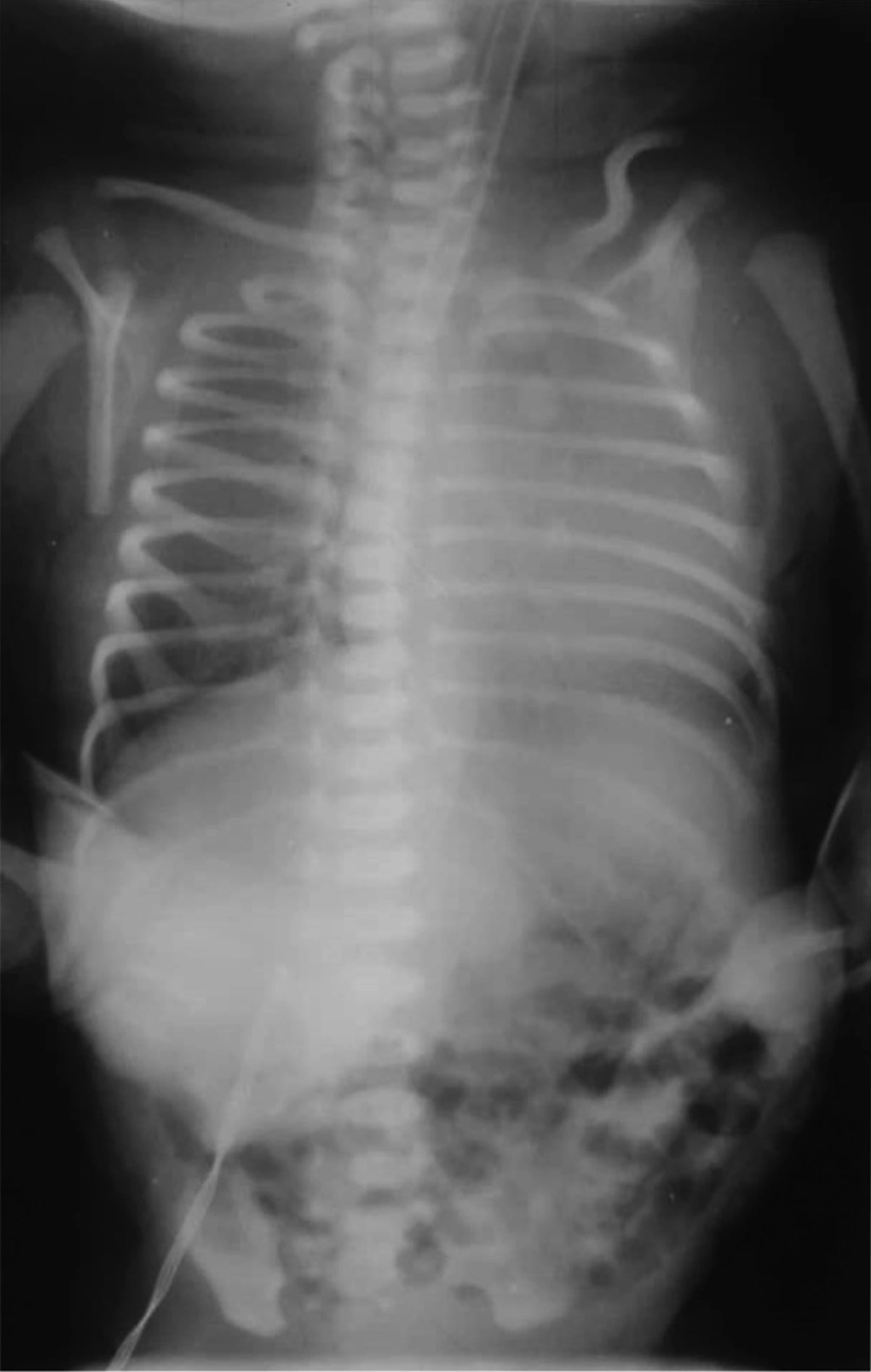
The second CXR, four hours after injection of the second dose of surfactant, showed mild hyperinflation that was decreased in comparison to previous CXR (Figure 2).
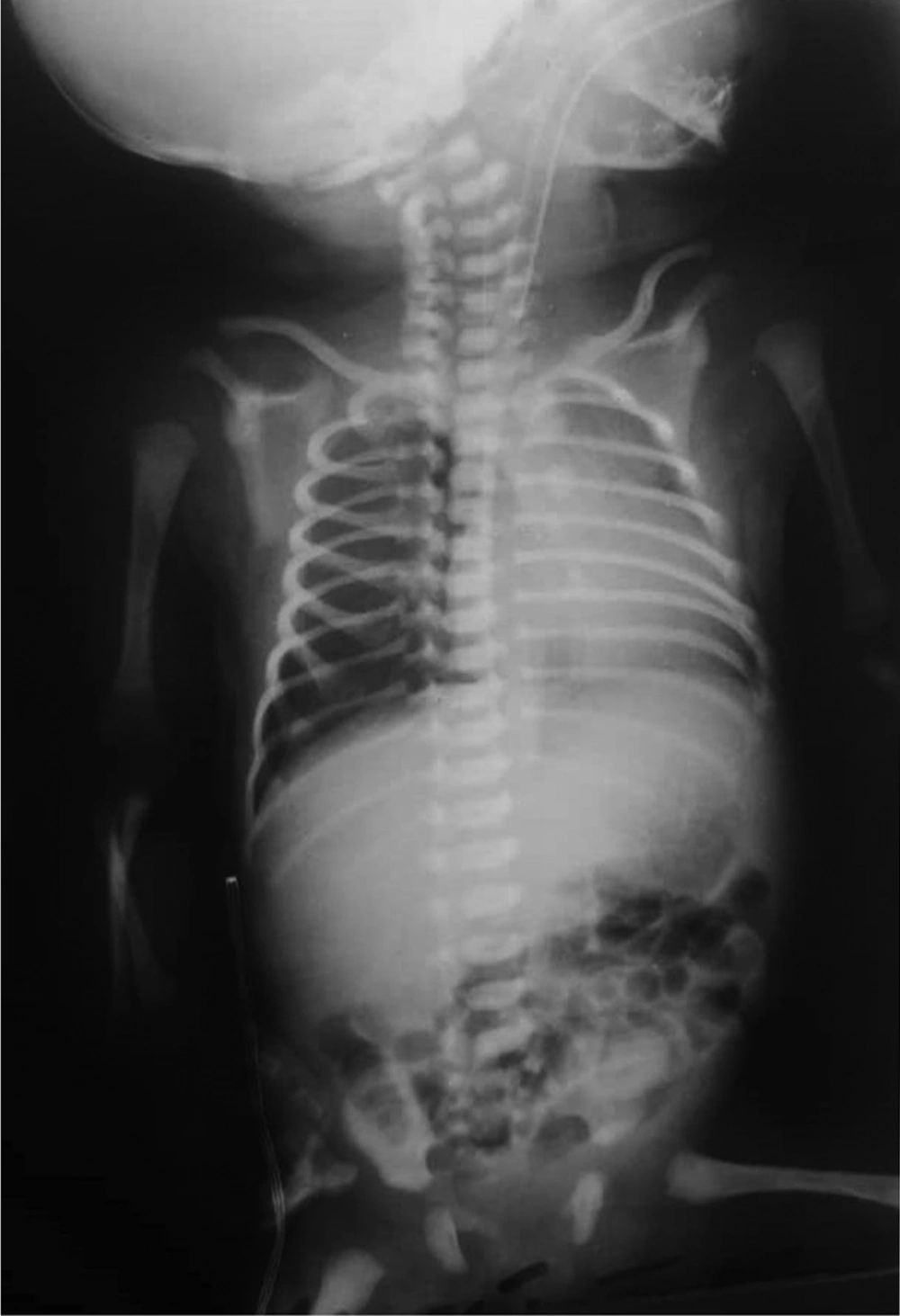
The third CXR, on the fifth day of birth: the lung tissue, had a normal pattern (Figure 3).
3. Discussion
We reported the case of a newborn in Iran with the diagnosis of SARS-COV-2 based on clinical symptoms, mother’s history, and positive rRT-PCR test for the mother and the neonate. The clinical manifestations of the mother were severe, and there was fatal distress that led to the preterm labor and emergency cesarean section. SARS-COV-2 infection in the mother could have caused hypoxemia and increased risk of birth asphyxia and premature birth (5). However, changes in the fetal heart rate pattern may also be a sign of severe maternal respiratory distress (12). A rapid review reported preterm delivery occurred in 47% of pregnant women with SARS-COV-2, which can put considerable pressure on maternal and neonatal care services (13).
The neonate’s test for SARS-COV-2 was positive on the fourth day of birth. Because of our resource limitation, SARS-COV-2 test for neonates is performed if the mother’s test is positive. Therefore, immediately after the positive report of the mother’s test, the neonate was tested for SARS-COV-2.
Different transmission routes might be considered for neonatal infection, including: (1) vertical transmission from the mother to the fetus during pregnancy; (2) contact between maternal blood and body fluid with the fetus during a cesarean; and (3) other contact transmission of newborns in the hospital.
In our case, the mother had some symptoms related to SARS-COV-2 from approximately one week before admission and an exposure to a suspected SARS-COV-2 case in her family. Although due to the limitations of diagnostic testing for SARS-COV-2 in the hospital, diagnostic testing of amniotic fluid, cord blood, and the placenta was not performed, but intrauterine vertical transmission could not be ruled out for the presented case. We tried to meet the isolation strategy for the baby, but it was not possible to rule out the other contact transmission.
In the first case report of a neonate with pharyngeal swabs tested positive by rRT-PCR assay 36 hours after birth, introduced by Wang et al. in Wuhan, China, the results of nucleic acid testing of the placenta and cord blood were negative, but the authors stated that the possibility of vertical intrauterine transmission of SARS-COV-2 could not be ruled out in that case (7).
Zhu et al. also reported the clinical manifestations and health outcomes of nine pregnant mothers with SARS-COV-2 and their neonates in China. Early symptoms of neonates included fever, respiratory distress, gastro-intestinal symptoms, vomiting, and increased heart rate. The pharyngeal swab sample of the neonates was negative for 2019-nCoV one to nine days after birth. However, false negative results should be considered, and the authors concluded perinatal 2019-nCoV infection could cause problems such as fetal distress, preterm labor, respiratory distress, and even death in neonates. Another result in that study was that early intravenous injection of immunoglobulin may reduce neonatal morbidity and mortality (5). We also used IVIG for the baby, and we believe it was helpful for the patient's improvement, but further studies are needed for this issue.
Chen also examined the clinical features and potential of intrauterine vertical transmission of SARS-COV-2 infection in nine pregnant women in China. The study reported no evidence of intrauterine infection caused by vertical transmission of SARS-COV-2 in late pregnancy. Just one neonate had a mild increase in myocardial enzymes on the day of birth without any clinical symptoms (9).
Li et al. in a study assessed 16 confirmed SARS-COV-2 pregnant women and 18 suspected cases and reported no SARS-COV-2 infection in their neonates. Also, severe complications of SARS-COV-2 pneumonia were not seen in any of the mothers and neonates (14).
Schwartz reported no evidence of intrauterine transmission in pregnant women with SARS-COV-2 (15). In Fan et al.’s study, also SARS-COV-2 was not detected in the two studied neonates (16).
Dong reported a newborn with elevated SARS-CoV-2 IgM antibodies (probably due to infection in utero) with a COVID-19 infected mother. However the increased IgM suggested the infection in utero, the results from repeated RT-PCR tests in neonate was negative and was difficult to explain for authors (17).
Zeng reported 33 neonates born to mothers with SARS-CoV-2 that nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life in 3 neonates (18).
Yu et al. presented seven cases of COVID-19 in late pregnancy that 4 neonates without any symptoms were not tested and 3 neonates remained for observation and were tested for SARS-CoV-2. Test of one neonate was positive at 36 hours after birth (19).
A summary of the data derived from the literature review related to neonatal cases of mothers with SARS-COV-2 infection is presented in Table 2.
| Author | Number of Neonatal Cases | Specimens for SARS-COV-2 Tests | Clinical Findings | Chest X-Ray |
|---|---|---|---|---|
| Wong et al. (7) | 1 | Cord blood, placenta and neonatal throat | Vomiting once after feeding formula | Lung texture with no abnormalities in heart and palate |
| Zhu et al. (5) | 10 | Neonatal throat | Fever, shortness of breath, rapid heart rate, vomiting feeding intolerance, bloating, refusing milk, and gastric bleeding | Infections, neonatal respiratory distress syndrome and pneumothorax |
| Chen et al. (9) | 9 | Amniotic fluid, cord blood and neonatal throat | No events of neonatal asphyxia | - |
| Li et al. (14) | 17 | Neonatal throat | No events of severe neonatal asphyxia | - |
| Fan et al. (16) | 2 | Amniotic fluid, placenta tissues, umbilical cord blood and newborn’s Nasopharyngeal | Fever and abdominal distension | Haziness in lung field without patchy consolidation |
| Dong et al. (17) | 1 | Nasopharyngeal | No symptom | - |
| Zeng et al. (18) | 33 | Nasopharyngeal and anal | Asphyxia, fever, shortness of breath, feeding intolerance, cyanosis | Radiographic findings were nonspecific |
| Yu et al. (19) | 7 | Neonatal throat | mild shortness of breath symptoms | mild pulmonary infection |
Overall some of the previous studies evaluated the presence of SARS-COV-2 in the amniotic fluid, placenta tissues, umbilical cord blood, newborn’s nasopharyngeal. Some studies reported positive rRT-PCR and some of them reported negative rRT-PCR however false negative result for rRT-PCR should be considered because of viral loud or insufficient collecting of samples (20).
Chinese consensus on the prevention and control of SARS-COV-2 during the prenatal and neonatal period recommended that delayed cord closure be avoided. Contact between mother and baby is not recommended. Physicians and nurses who care for mothers and neonates suspected of SARS-COV-2 should have appropriate protective equipment (21).
In some studies, it is mentioned that SARS-COV-2 disease can cause acute cardiac injury (3, 22). What makes the presented case different from the newborns reported in other studies was the manifestations of congenital myocarditis, which, according to the reports of SARS-COV-2 cases with myocarditis, predispose them to fetal involvement during pregnancy. However, a negative troponin does not exclude the diagnosis of myocarditis (23, 24). In our case, progressive manifestations in the echocardiogram included elevated pulmonary pressure and decreased EF despite relieving respiratory distress and extubation, and response to IVIG and Millerine was in favor of myocarditis.
In Wang et al.’s study on a cohort of 138 patients with SARS-COV-2 hospitalized in China, cardiac injury (increased troponin, abnormality in electrocardiogram, or echocardiography) was reported in 7.2% of the patients (25). The case presented by Zeng et al. was a 63-year-old man with SARS-COV-2 in China who had increased troponin and myocardial dyskinesia with decreased left ventricular EF on echocardiography who was treated with a diagnosis of fulminant myocarditis (26). Hu et al. also presented the case of a 37-year-old man diagnosed with coronavirus fulminant myocarditis and suggested that early treatment with immunoglobulin therapy, glucocorticoid, and anti-inflammatory therapy was important in the management of this case (27). However, mechanisms that may explain cardiac involvement in SARS-COV-2 are currently unclear and need more investigations (28). Guo et al. reported inflammation may be a potential mechanism for myocardial injury for patients with SARS-COV-2 (29).
Since all aspects of SARS-COV-2 infection have not yet been identified, the consequences can be complex and difficult to control in the mother and neonate if the health services are not prepared. Therefore, in addition to health education and prevention, careful evaluations, and appropriate interventions through a multi-disciplinary team (12) for the care of pregnant women and neonates based on evidence are essential (30).
We believe that our case report can be helpful for understanding the clinical and paraclinical characteristics of neonates with SARS-COV-2, especially with myocarditis. However, further studies are needed to prove the possibility of intrauterine vertical transmission of SARS-COV-2 infection from the mother to the fetus and to determine the mechanism of cardiac injury in SARS-COV-2 infection.
3.1. Conclusions
The presented case was a neonate with confirmed SARS-COV-2 infection that had respiratory distress and manifestations of myocarditis at birth and was improved with care and treatment. Given the possibility of cardiac injury in SARS-COV-2 disease and manifestation of congenital myocarditis in our case, maternal vertical transmission of SARS-COV-2 could be considered.