1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) known as COVID-19, has become an unprecedented global pandemic. Iran reported its first confirmed cases of SARS-CoV-2 infection on 19 February 2020 (1), and since then, the infection rapidly surged to more than 140000 in three months and the mortality rate reached more than 7000 (2). In Tehran, the capital city, until 30 May 2020, more than 5000 confirmed cases have been reported (3).
Our pediatric hematopoietic stem cell transplant (HSCT) department, as the first and the most referred national center admitting different categories of pediatric patients needing HSCT, is embedded within a tertiary care hospital, affiliated to Tehran University of Medical Sciences (TUMS). As a referral center, our division provides HSCT for almost 100 pediatric patients per year, including high risk and complicated HSCTs from unrelated and haploidentical donors, and almost 70 patients per week receive outpatient visits in our pre- and post-HSCT clinics. Considering the central role of our HSCT unit, only a week after the announcement of the outbreak in the country, we had an expert session to modify the working guidelines and to develop new rules and regulations for HSCT recipients and donors to reduce the risk of infection in patients as well as staff. We considered national travel and accommodation restrictions, limitations in health care resources and in diagnostic and therapeutic modalities, the worries of patients’ families and caregivers, and also the concerns about the availability of post-HSCT prerequisites such as blood products, ICU care, and rehabilitation facilities. We took in to account the accessible published guidelines (4-6) from HSCT authorities around the world and modified them according to our circumstance as discussed below.
2. Arguments
2.1. The Plans We Made
We planned to disinfect the ward with 0.05% sodium hypochlorite (dilution 1:100), and for surfaces that may be damaged by sodium hypochlorite, ethanol-based products were used (at least 70%). We trained the staff about hand hygiene practices, social distance cautions, and wearing surgical masks while working in the unit. The employees were notified that if they happened to have symptoms related to COVID-19 (fever, cough, shortness of breath, anosmia) or to have close contact with a COVID-19 patient, they should leave the unit and be manage based on the national guideline of diagnosis and treatment of COVID-19. We restricted people’s commute into the unit and reduced non-essential staff & student contacts with inpatients.
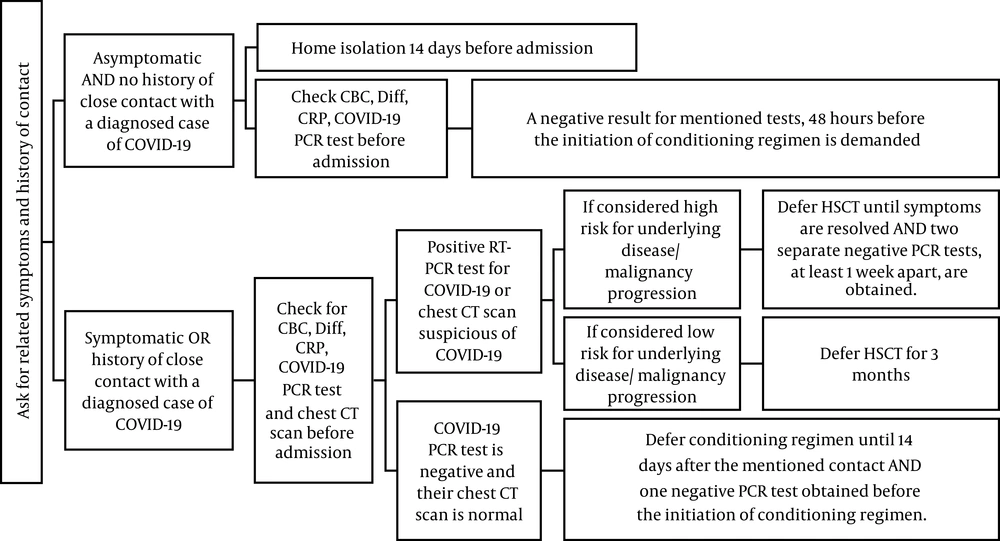
In the pre-HSCT clinic, we planned to cancel most non-malignant indications for HSCTs except cases of transfusion-dependent acquired and inherited bone marrow failures, severe combined immunodeficiency (SCID), and osteopetrosis. We also planned to defer most of the low-risk malignant candidates for HSCT (7, 8), but we planned to continue the HSCT process for cases of neuroblastoma and acute leukemia. For patients who urgently needed HSCT and those with other criteria for HSCT, we planned to approach according to Figure 1.
Only one companion is allowed to stay with the respective patient during the treatment course, and s/he should be asymptomatic, and a negative COVID-19 PCR test should be presented before admission. No other visitor is allowed in the ward since the outbreak.
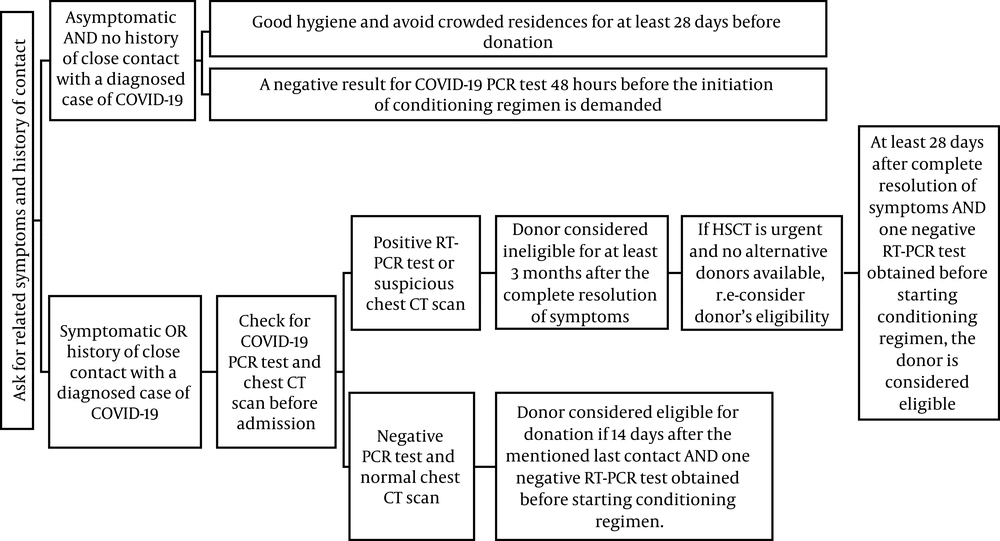
In the section for donor selection, as the operation room in our hospital is shared between different specialties, we planned not to use bone marrow harvesting as the source for providing stem cells due to the possibilities of donors’ exposure to COVID-19 while moving to the general operating room. Although the Food and Drug Administration (FDA) has reported that coronaviruses are not transfusion‐transmittable infections, we planned to select our donors according to Figure 2.
In our post-HSCT clinic, the rooms’ windows were kept open for air ventilation, and only one companion at a time was allowed to enter the examination room with the patient. We planned to use virtual healthcare options by online medical platforms and teleconsultations, when safe and feasible. For those who came to the clinic, we indorsed to wear facial masks, keep at least one meter distance with others, and to wash their hands before entering and after exiting the clinic. The clinicians were also indulged to wear facial masks, disposable long-sleeved gown, and washing their hands before and after examining each patient.
We decided to treat patients presenting with post-transplant complications, out-patiently as much as possible, and to continue the follow-up appointments by phone (except when the physical examination would be necessary).
We organized to use triage from our emergency room (ER) division; if the patient presented with problems needing admission other than symptoms suggestive of COVID-19, we decided to check COVID-19 PCR and chest CT scan and if either were suggestive of COVID-19, the patient would be admitted in the general wards specialized for COVID-19 patients. If negative COVID-19 PCR and normal chest CT scan were obtained, then the patient would be admitted to our post-HSCT ward, where all patients and their helpers are obliged to wear surgery facial masks and are discouraged to leave the ward unless necessary.
2.2. Encountered Problems
When the COVID-19 outbreak was officially announced in Iran, we had eight patients admitted in our HSCT ward whose characteristics are summarized in Table 1. We continued their typical care but at the time of discharge, supplementary to their usual medications, according to initial positive data form multiple small studies (9-11), we administered hydroxychloroquine sulfate (pediatric dose: 6.5 mg/kg, not to exceed 400 mg) every 3 weeks until the pandemic lapse, as a prophylaxis for COVID-19. However, recent studies have questioned the role of the drug and highlighted its cardiotoxicity. Regarding that enough evidence are not available about the role of hydroxychloroquine in preventing COVID-19, we stopped its administration (12, 13). It worth mentioning that meanwhile, we did not observe any complication or adverse effect after its administration.
| Underlying Disease | Sex | Age, y | Donor | HSCT Type | Duration of Admission After HSCT, d | |
|---|---|---|---|---|---|---|
| 1 | Osteopetrosis | Male | 2 | Full-matched aunt | Allogeneic | 22 |
| 2 | FA | Male | 9 | Full -matched sibling | Allogeneic | 24 |
| 3 | FA | Male | 6 | Full -matched sibling | Allogeneic | 23 |
| 4 | TM | Male | 18 | Full -matched mother | Allogeneic | 21 |
| 5 | ALL | Male | 4 | Full -matched sibling | Allogeneic | 16 |
| 6 | ALL | Male | 18 | Full -matched sibling | Allogeneic | 21 |
| 7 | SAA | Male | 16 | Full -matched sibling | Allogeneic | 40 |
| 8 | Neuroblastoma | Male | 18 | - | Autologous | 17 |
Abbreviations: ALL, acute lymphoblastic leukemia; FA, Fanconi anemia; SAA, severe aplastic anemia; TM, thalassemia major.
Unfortunately, due to international flight canceling and restrictions, we had no choice except to cancel the urgent HSCTs from unrelated foreign donors in a proportion of our patients, including seven cases of acute leukemia, a case of myelodysplastic syndrome (MDS) secondary to Fanconi anemia (FA), a transfusion-dependent FA, and a transfusion-dependent severe aplastic anemia (SAA). Even one of our Iranian donors refused to donate stem cells to a 9-year-old boy with acute lymphoblastic leukemia (ALL), due to the restrictions on traveling between cities and the phobia of infecting with COVID-19 during the hospital stay. These patients and their parents became very distressed, and we provided virtual counseling and mental-health assistance from our psychiatric team and assured them to be notified as soon as stem cell transferring became available. Since the onset of the COVID-19 epidemic, the number of patients admitted in the HSCT ward dropped substantially, and we only admitted thirteen patients for the last three months, whose data are summarized in Table 2. It is important to note that, as recommended in our guideline, we tested all the recipients, donors, and caregivers for COVID-19 PCR, and all test results were negative before admission. In the last three months, we have had 3 positive results of COVID-19 PCR in our center, but they were all in the adult age category and we did not come across any positive result in our pediatric patients.
| Underlying Disease | Sex | Age, | Donor | HSCT Type | Duration of ADmission After HSCT, d | |
|---|---|---|---|---|---|---|
| 1 | Pre B ALL in CR2 | Female | 7 | Full-matched sibling | Allogeneic | 26 |
| 2 | AML in CR 1 | Female | 10 | Haploidentical | Allogeneic | 33 |
| 3 | Pre B ALL in CR2 | Male | 10 | Haploidentical | Allogeneic | 24 |
| 4 | Pre B ALL in CR2 | Female | 16 | Full-matched sibling | Allogeneic | 22 |
| 5 | SAA | Male | 13 | Full-matched mother | Allogeneic | 20 |
| 6 | FA | Female | 8 | Full-matched sibling | Allogeneic | 24 |
| 7 | AML in CR2 | Female | 10 | Haploidentical | Allogeneic | 44 |
| 8 | ALL in CR3 | Female | 14 | Haploidentical | Allogeneic | 17 |
| 9 | Neuroblastoma in CR2 | Male | 15 | - | Autologous | 18 |
| 10 | Neuroblastoma in CR1 | Male | 4 | - | Autologous | 14 (still admitted) |
| 11 | Ph+ Pre B ALL in CR1 | Male | 16 | Full-matched sibling | Allogeneic | 15 (still admitted) |
| 12 | AML in CR1 | Female | 12 | Full-matched sibling | Allogeneic | 7 (still admitted) |
| 13 | Pre B ALL in CR2 | Male | 10 | Full-matched sibling | Allogeneic | 4 (still admitted) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CR, complete remission; FA, Fanconi anemia; SAA, severe aplastic anemia; TM, thalassemia major.
In the post-HSCT clinic, we faced three complicated patients needing admission; the first case was a 6-year-old boy with FA who had undergone HSCT from his HLA-matched sibling 41 days before and he presented with seizure. He did not have any respiratory symptoms, so was admitted to the ER, checked COVID-19 PCR, which came back negative, and then we admitted him in the post-HSCT ward. His brain MRI indicated evidence of posterior reversible encephalopathy syndrome (PRES), and so his cyclosporine, which was administered as GVHD prophylaxis was discontinued, and he was discharged after 2 days with a good condition.
The second case was a 14-year-old boy who had undergone haploidentical HSCT due to ALL in CR3, 65 days before, he presented with hemorrhagic cystitis, fever, and cough; COVID-19 PCR and chest CT scan plus infectious disease specialist consultation were requested while admitting in the ER. According to the radiologist report, his PCR result came back negative, and his chest CT scan was in favor of fungal infection. So he was admitted in post-HSCT ward, antifungal therapy, and bladder irrigation were administered, and he is still hospitalized due to the continuance of hemorrhagic cystitis complications. Worth mentioning, his pulmonary fungal infection is resolved.
The third case was a 16-years-old boy with thalassemia major who had undergone allogeneic HSCT from his HLA-matched mother, 70 days before. He presented with profuse watery diarrhea. Although the most possible diagnosis was gastrointestinal GVHD, COVID-19 PCR, and chest CT scan were requested, which results came back normal, and then he was admitted in the post-HSCT ward. He responded to corticosteroid therapy and was discharged with a good condition after 5 days.
3. Discussion
Limited data are available on the children who have been affected by COVID-19, so it is difficult to reach a reliable conclusion about the most susceptible pediatric population for SARS-CoV-2 infection and death. Inclusively, the data on COVID-19 in children is reassuring, infection rates have been reported to be lower than the general population, and most infected children have been asymptomatic and did not require invasive support (14). On the other hand, COVID-19 has imposed many challenges and constraints on patients undergoing HSCT. In patients who received the HSACT process, T- and B-lymphocytes and immune memory are lost, and mucocutaneous barriers are damaged. Thus, they are highly susceptible to opportunistic infections (15). Community-acquired viral respiratory infections can occur in more than half of HSCT recipients and so, CDC has recommended to strictly follow infection control measures, such as using protective gear and isolation (15, 16). Little is known about the effect of COVID-19 on pediatrics with HSCT. In a report from two cancer centers in New York, only two post-HSCT pediatric patients have reported being COVID-19 positive, and most of their patients have had mild disease, parallel to foregoing reports of pediatric patients from China and Europe (17).
4. Conclusions
Three months after the beginning of the outbreak, no evidence of COVID-19 has been detected in our pediatric HSCT ward so far. Unlike many centers who initially underestimated the risk of COVID-19 in pediatric patients, we promptly adopted preventive policies and strictly adhered to our plans. This has given us the proudness of being still successfully active in the field despite all the impediments and obstacles which came into our path due to the COVID-19 outbreak. We are now more powerful and experienced in dealing with future potential difficulties.
As a final point, we are not aware of the duration or outcome of the pandemic, but we know for sure that our vulnerable patients are being troubled, both directly and indirectly. So we are intended to learn from our experiences and figure out the best strategies to minimize the possible harms.