1. Context
In December 2019, an increasing number of unknown viral pneumonia cases were reported from Wuhan, Hubei Province, China. Soon after, more similar cases were reported. By December 31, 2019, 27 cases of pneumonia with an unknown etiology were reported to the World Health Organization (WHO). The pathogen was identified as a novel coronavirus in early January, and finally, on January 30, 2020, the WHO declared the novel coronavirus a public health emergency of international concern (1-3). As of April 18, 2020, more than 2,275,401 cases of 2019-nCoV infection were confirmed worldwide, with more than 150,000 deaths and 210 involved countries. Early cases showed a positive history of a common zoonotic source of infection, i.e., the Huanan Seafood Wholesale Market, while further reports of infection in patients without any similar history were suggestive of a new route for spreading infection (3, 4).
Of more than 72,000 confirmed cases of 2019-nCoV up to February 11 in the main land of China, 590 cases were under the age of 19 years, which were mostly asymptomatic or had mild clinical presentation. This explains the need for accurate case detection in children (3). Although these patients do not present typical clinical features of infection, they have positive viral nucleic acid test results and can play an important role in spreading the infection in populations (3). However, there is a substantial informational gap in the epidemiology, pathology, and clinical presentation of Coronavirus Disease 2019 (COVID-19) in pediatric patients. It is still unknown whether the lower severity of the disease in the pediatric age group is due to the nature of the virus, or it is because of better immunity during childhood. Patients under 19-years-old comprise an important group of asymptomatic carriers that need to be more precisely and wisely studied. Here, we reviewed the current situation of pediatric patients infected with COVID-19 in comparison with Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) patients.
2. Evidence Acquisition
In this review, research articles published before April 18, 2020, were reviewed to understand the clinical characteristics of COVID-19 in children. The WHO (https://www.who. int/) and CDC (Centers for Disease Control and Prevention, https://www.cdc.gov/) websites were also reviewed to find eligible studies, besides articles extracted from PubMed, Scopus, and Google Scholar. We studied English articles with keywords, including children, pediatric, COVID-19, SARS, MERS, and their equivalents.
3. Results
3.1. Severe Acute Respiratory Syndrome
The novel coronavirus 2019 is a new member of the family Coronaviridea and the genera beta coronavirus (1, 2). In November 2002, SARS cases were reported for the first time from China, and soon after, the WHO enforced a global effort to identify the causative agent of SARS. By April, SARS coronavirus (SARS-CoV) was identified and characterized as a new coronavirus. Although the passengers who visited Fushin, China, were the main transporters of the virus to other countries, more than 30% of the infected patients were health care workers, suggesting a nosocomial route of transmission as the main route of transmission for SARS coronavirus in adults. After more than 8,000 people were infected, and more than 700 deaths occurred in 27 countries by July 2003, and no more cases were reported, the WHO declared that the SARS outbreak was over (5-9). Children were affected at a rate of 5% - 7% in the SARS outbreak (10), and only 135 pediatric cases of SARS have been well reported in the literature (11). Among probable cases of SARS, more than 80% had a history of close contact with confirmed cases of SARS, and only 10% had nosocomial infections (11). According to such data from patients, Bitnun et al. suggested that in the context of SARS-CoV epidemics, every child with fever and positive history of close contact with confirmed cases with or without respiratory symptoms should be investigated for infection with SARS-CoV (12). Secondary infection from confirmed cases to other children and adults was less likely in young patients, while it was common among adult patients (13-15). However, the report of three adults and one child infected following close contact with an 11-year-old patient (5, 7) assured the possibility of virus transmission from children to other people (11). Female children were more involved, and patients younger than 12 years of age only showed mild symptoms and were less likely (2% according to available data) to develop severe conditions (11).
Patients older than 12 years had similar clinical presentations to adults, and 11% of those who developed severe conditions but survived were pediatric cases (≤ 18-years-old) (11). Fever, cough, nausea, and vomiting were the most common symptoms among pediatric cases (11). Other symptoms included myalgia, chills, and headache, which were more common among children older than 12 years (11). Diarrhea, although was common among adults, occurred only in 23% of pediatric patients (11, 16). Radiographic findings, including patchy infiltrates, opacities, and areas of consolidation with multifocal lesions, predominantly in lower lobes, were detected in infected pediatrics from the early onset of symptoms, which was in contrast to reports from adult patients (11, 17). The most common laboratory test findings among pediatric patients included lymphopenia, leukopenia, thrombocytopenia, and elevated levels of lactate dehydrogenase (LDH) and alanine aminotransferase (ALT) (17-19). However, lymphopenia was not detected in a group of children who did not receive corticosteroids (12, 20). In children, the most sensitive test for the confirmation of infection was Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for the RNA of SARS-CoV in nasal aspirate or Broncho alveolar lavage specimens (11, 21). It was notable that the appropriate timing of sampling was most important in the positivity of the test. The best time for sampling was when there was a high viral load in the patient, although the viral load in children had never reached the level of adult patients (12, 20). The RNA of the virus was detected in 48% of young patients within five to seven days of the symptom onset (12, 21). Since there was still a lack of case definition with high sensitivity and specificity for patient detection, laboratory tests were of most accuracy for the diagnosis of SARS in pediatrics (11). Thus, SARS seems to present with mild symptoms in young patients, and only may patients with severe disease develop further complications, including exercise intolerance and residual lesions in CT scan (12, 20). The SARS infection should be differentiated from other respiratory viral infections, including influenza virus A, parainfluenza virus 3, respiratory syncytial virus, varicella zoster virus, and rota virus, and also streptococcus pneumonia (12). Patients without SARS-CoV infection rarely show abnormalities in chest radiography; however, all children suspected for SARS-CoV infection should be investigated for co-infections, especially with influenza viruses (12, 22).
3.2. Middle East Respiratory Syndrome
In June 2012, a man with evidence of viral pneumonia developed multi-organ failure and died in Saudi Arabia. Soon after, the causative agent was detected and reported as a new coronavirus, Middle East Respiratory Syndrome coronavirus (MERS-CoV). Middle East Respiratory Syndrome had been one of the challenging viral infections of the respiratory tract among hospitalized patients all over the world. By January 2018, 2,143 cases were confirmed for MERS-CoV infection, with more than 700 deaths (23-28). According to the WHO report, 36 cases were under 19-years-old, which suggested that pediatrics were affected with a rate of 2% in the MERS outbreak (10, 29-31). MERS-CoV involved female children more than male children (29). Similar to SARS, most of the cases had a positive history of household contact (29, 32). Healthcare workers are at a higher risk of infection with coronaviruses, which is mostly due to the production of bio-aerosols (33, 34). Since most pediatric cases only developed mild symptoms and were rarely in need of hospital visits or admissions, the rates of contact and infection transmission to healthcare workers from children and vice versa were much lower than that occurred by adults (35). However, sporadic MERS-CoV infection was seen in patients with no past medical or contact history (36). Almost all of the infected children were asymptomatic or had mild symptoms. Available data suggest that 56% (9/15) of infected cases had no specific symptoms in primary stages and only 37% (6/15) developed mild symptoms, while 18% (3/15) of the patients with background diseases (cardiac disease, nephrotic syndrome, and cystic fibrosis) developed severe conditions, two-thirds of whom died (29, 32, 36-40). Fever and mild respiratory symptoms were common among symptomatic cases, while severe respiratory distress only happened in patients with comorbidities (29). It was notable that only pediatric cases with MERS who had associated comorbidities developed renal failure and had poor outcomes (29, 32). Radiologic findings of MERS-CoV in children showed bilateral diffuse infiltrates (36). Other infectious agents such as influenza virus A, rhinoviruses, and enteroviruses were common among patients with a clinical diagnosis of MERS, which should be ruled out in suspected cases of MERS (41). Due to the mild clinical picture of MERS and SARS in children, there is only limited literature available for pediatricians, while the early diagnosis of infected pediatrics is of high importance in controlling the transmission of infection.
3.3. Coronavirus Disease 2019
The pediatric population is infected with COVID-19 to a similar extent to the adult population when contact with a case of COVID-19 occurs (42). However, initial epidemiological studies showed that less than 3% of confirmed cases were under 19-years-old (3). As of February 19, 2020, among 391 confirmed cases of COVID-19 in Shenzhen, China, 84% had a positive history of at least one close contact with a confirmed case (42, 43). Similar to SARS and MERS, close contact plays the most important role in the transmission of the new coronavirus among pediatric patients (44). Some cases were asymptomatic at the time of diagnosis and were tested for the viral RNA during screening for close contacts with previously confirmed cases (4, 22, 45, 46). The detection of case clusters in an early stage after a patient is confirmed for 2019-nCoV infection helps in narrowing the spread of the virus. Of 1,184 case clusters detected in China, 64% were reported for familial households. In Singapore, the study of 18 hospitalized patients with PCR-confirmed 2019-nCoV infection, two family clusters showed exposure to four infected cases, affirming the important role of family clusters in the spread of COVID-19 (3, 47). Similar to SARS and MERS outbreaks, female pediatrics are more involved in the COVID-19 pandemic (4, 22, 45, 46). The youngest patient confirmed for infection with COVID-19 was a neonate aged less than one-week-old, which is younger than the youngest SARS and MERS patients, who aged nine and seven months, respectively (10, 45), suggesting the wide spectrum of ages that can be infected with the novel coronavirus. The majority of pediatric cases are asymptomatic or have mild symptoms (3) and rarely progress into ARDS stage and multi-organ failure (22, 46, 48). The incubation period is 2 - 10 days among the symptomatic patients (22). The most common symptoms among reported cases are fever and cough, although sore throat, stuffy nose, sneezing, and rhinorrhea have also been reported in some cases (22, 46). The most common clinical presentations and imaging results among pediatric cases of COVID-19 are listed in Table 1. While a rate of 20% - 30% deaths is reported for patients older than 60 years, only one death in pediatric patients has been reported in China (3, 49). Nasopharyngeal swabs for nucleic acids of COVID-19 virus using PCR become negative within 6-22 days of symptom onset in all patients (22, 46). The virus also has been detected in other secretions of patients. The RNA of the virus is detectable in the fecal samples of patients even within 18-30 days after the onset of symptoms while they may have no gastrointestinal symptoms (22, 50). Evidence of viremia was reported for only one case in Singapore (50), but the RNA of the virus has still not been detected in the urine (22). Co-infection with influenza virus and rhinoviruses was evaluated among some cases, but no patient has been detected with co-infection with influenza virus (22).
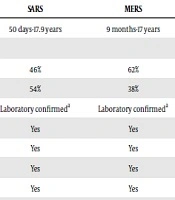
| SARS | MERS | COVID-19 | |
|---|---|---|---|
| Age range | 50 days-17.9 years | 9 months-17 years | 1 day-18 years |
| Gender | |||
| Male | 46% | 62% | 56.60% |
| Female | 54% | 38% | 43.40% |
| Case definition | Laboratory confirmeda | Laboratory confirmeda | Laboratory confirmeda |
| Fever > 38 | Yes | Yes | Yesb |
| Cough | Yes | Yes | Yes |
| Myalgia | Yes | Yes | Less common |
| Malaise | Yes | Yes | Less common |
| Rhinorrhea | 22% | Less common | 8% - 13% |
| Dyspnea/tachypnoea/shortness of breath | Yes | Yes | Yes |
| Pharyngeal congestion/sore throat | Yes, sore throat | Less common | Yes, pharyngeal congestion |
| Vomiting/nausea | Yes | Yes | Yes |
| Diarrhea/constipation | Yes | Less common | Yes |
| Headache | Yes | Less common | Yes |
| Mild/asymptomatic | NA | 47% | More than 50% |
| Radiographic signs of pneumonia at onset of illness | 48% | 100% Bilateral diffused infiltrate | 22% Unilateral pneumonia, 56% Bilateral pneumonia (patchy shadows or lung consolidations) in chest CT |
| Radiographic changes during the course of illness | 97% | NA | 75% Multiple patch-like shadows, 75% GGO, 12% White lung appearance, 12% Pleural effusion, 12% Multiple mottling |
| Leukopenia | 47% | NA | Less than 15% |
| Lymphopenia | 46% | NA | Common |
| Increased CRP | NA | Yes | Yes |
| Increased LDH | 63% | NA | Less common |
| ICU admission | 10% | 18% | Less than 5% |
| Case fatality rate | 9.60% | 35.50% | 3-3.5% |
| Case fatality rate with comorbidities (%) | 46% | 60% | 73% |
| Supplementary oxygen therapy | 27% | 25% | Less common |
Abbreviations: GGO, ground glass opacity; NA, not available
aConfirmed infection by PCR and/or ELISA in the main land of China.
bWe refused to use exact figures due to the lack of wide spectrum retrospective studies on the clinical presentations of children infected with COVID-19 in the literature and the daily increasing number of patients worldwide.
3.4. Transmission of COVID-19
The children-to-adult transmission of COVID-19 is possible (22); however, it has been a key question since the early days of the pandemic, and more epidemiological studies are needed to identify the role of children in the transmission of COVID-19 to other children or adults (51). One hypothesis for the lower severity of COVID-19 in the pediatric group is the non-efficient transmission of the virus, which may result from its specific molecular signaling and receptor molecules in children. A well-known example for the efficacy of transmission is the influenza virus. The H1N1 virus binds to its specific receptors in the upper respiratory tract and causes mild symptoms in patients while the H7N9 virus binds to its specific receptors in the lower respiratory tract and causes more severe symptoms than does H1N1 (52). In the case of coronaviruses, SARS-CoV and NL63-CoV can cause different severities of disease in patients, while they bind to the same receptor, Angiotensin Converting Enzyme 2 (ACE 2) (52). However, more data on the molecular pathogenesis of COVID-19 in children are needed to confirm whether the severity of the disease is dependent on the molecular pathogenesis of the virus or not.
3.5. Angiotensin Converting Enzyme 2 Dilemma
The COVID-19 virus has recently been known to bind to Angiotensin Converting Enzyme 2 (ACE2), which was previously known as the specific receptor for SARS-CoV (2, 28, 53-55). The binding of SARS-CoV to ACE2 is responsible for lung injury during SARS (56). It is well known that when SARS-CoV spike glycoproteins bind to this receptor, ACE2 is downregulated and results in the over production of Angiotensin 2 (AG2) (28). Over-stimulation of type 1a Angiotensin 2 receptors by AG2 increases pulmonary vessels permeability and further lung damage (57). It is suggested that the rapid progression of severe respiratory symptoms of SARS-CoV in patients is associated with higher expression of its receptor, ACE2 (58, 59). The expression of ACE2 studied in several adult tissues showed different expressions in different tissues (58). A small proportion of alveolar type 2 (AT2) cells of the human lungs has been recently known to highly express ACE2 (60). Other cells of human lungs that express ACE2 with lower ratios are AT1 cells, airway epithelial cells, fibroblasts, endothelial cells, and macrophages (60). The expression of ACE2 is more in adult males than in females, as seen in the epidemiological data of COVID-19, possibly leading to the higher involvement of adult males than the female population (3, 60). The age-dependent variation in host responses in ARDS was studied by Schouten et al. and it showed to be related to the ACE2 level in patients (61). However, there were significant variations in ACE2 activity among pediatric patients, which made it difficult to detect even small differences in the levels of ACE2 between pediatrics and adults (61). Another study showed that the expression of ACE2 increases in young male children as they grow up, while it decreases in females as puberty changes begin (62). According to the similarities between SARS-CoV and 2019-nCoV molecular pathways of pathogenesis, the demonstration of ACE2 expression and activity in the pediatric age group might show the molecular basis for the lower severity of COVID-19 in the pediatric group.
3.6. Multisystem Inflammatory Syndrome
Despite the lower severity of COVID-19 in the pediatric population, since May 2020 during the COVID-19 pandemic, pediatricians have faced critically ill cases presenting with fever, rash, and multi-organ failure similar to patients with Kawasaki disease or toxic shock syndrome (63, 64). The condition in children is called Multi-System Inflammatory Syndrome in Children (MIS-C). The association between COVID-19 and MIS-C is not fully understood, and it is not clear who is most at risk for this illness. One hypothesis on the pathogenesis of MIS-C is the relationship between the viral load and host response. In cases with low viral loads, interferon (IFN) response results in viral clearance and mild disease. In genetically susceptible hosts or high viral loads, IFN responses may delay due to virus replication. Subsequently, the cytokine storm may be seen before viral clearance (65).
3.7. Detection of COVID-19 in Children
The confirmation of suspected cases of COVID-19 is based on the Polymerase Chain Reaction (PCR) test to detect the RNA of the virus in samples taken from nasopharyngeal swabs or bronchoalveolar lavage (BAL). A positive test result means that the RNA of the 2019-nCoV virus is present in the nasopharyngeal mucosal membrane. The RNA of the virus was detected in blood samples of some severe cases of adult patients (66), which was assumed to be due to the severity of the disease (22). Viremia is reported in the pediatric group of patients, too. One infant was reported with a positive blood test for 2019-nCoV with no symptoms during hospital admission except an episode of 38.5ºC fever during the course of viremia, which got relieved after one hour with no medications (50). The RNA of the virus has also been detected in fecal samples of some confirmed cases of COVID-19 in the young age group (22). There is no report of diarrhea or other gastrointestinal symptoms in pediatric patients infected with 2019-nCoV (22), while diarrhea was common in SARS patients. Previous studies also reported high expression of ACE2 in smooth muscle cells and endothelium of vessels of the stomach, small intestine, and colon, as well as enterocytes of the small intestine (58). According to these reports, the GI tract seems to be a new site of replication for 2019-nCoV and emerges to establish more caution on the transmission of the virus through GI secretions of young age patients. Many epidemiologic studies should be done to examine the different aspects of COVID-19 and factors affecting COVID-19, especially in children.
4. Conclusions
In conclusion, the natural course of coronaviruses in children is still not defined and needs more precise studies seeking the nature and behavior of the virus among pediatric patients with the hope of discovering a cure for COVID-19. The low prevalence of COVID-19 in children may be due to the incomplete identification rather than resistance to the virus. SARS-CoV-2 has a wide range of transmission, clinical, and laboratory presentations in comparison with SARS and MERS, which has made it difficult to eradicate. As the pandemic is still ongoing around the globe, more pediatric cases may be reported in the near future with similar or different clinical manifestations and we need a high degree of suspicion for COVID-19 infection in the pediatric population.