1. Context
Sepsis and septic shock are significant healthcare problems worldwide to consider incidence, healthcare burden, death, and cost (1). Despite progression in the knowledge, diagnosis, and medical care for sepsis, it remains one of the most common causes of death in adults and children, challenging in the primary medical specialties. The disease burden is difficult to ascertain; however, the World Health Organization (WHO) estimates it as 30 million cases and 6 million deaths worldwide (2, 3).
Although a significant progression in understanding pathogenesis and sepsis treatment have been achieved in the last four decades, the current therapeutic modalities are not highly effective (4). Researchers have recognized two phases in the evolution of pathways leading to death due to sepsis, including the early hyperinflammatory phase with 10% mortality and the late immune suppression phase accounting for 30% of deaths (4). At least 40 trial sepsis researches directed to control sepsis’s early phase have been found unsuccessful (5, 6). Limited studies show that stem cells might be an alternative therapeutic approach. Here, the authors have reviewed the current progression in stem cell therapies for sepsis and septic shock.
2. Evidence Acquisition
Herein, we have reviewed and discussed current problems with sepsis management and stem cell therapy in sepsis, preclinical, experimental studies, and early-phase clinical trials using stem cells to treat sepsis.
2.1. Search Strategy
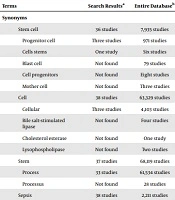
In the paper preparation, the authors searched electronic databases, including PubMed, Google Scholar, Web of Sciences, and the clinical trials website at the National Library of Medicine (NLM), for articles published between 2010 and 2020 (date of the last search: 30 January 2020). We searched databases using these keywords (alone and in combination): “sepsis”, “septic shock”, “children”, “stem cell”, “mesenchymal stem cell”, “therapy”, “treatment”, “animal model”, “murine models”, and “cell therapy”. A Google Scholar Alert was activated for keywords to receive notifications about newly published articles, primarily clinical trials, during the manuscript preparation.
2.2. Eligibility and Exclusion Criteria
The study included the articles types of sepsis-related systematic reviews, meta-analysis, and narrative reviews for stem cell therapies (Table 1). They were screened first by titles according to the relevancy criteria and categorized based on relevancy, types of articles, and publication year. The abstracts of selected articles were reviewed by three authors [Jafar Soltani (JS), Zeynep Burcin Gonen (ZBG), Gokcen Dinc (GD)] separately, and each relevant article re-categorized according to researcher specific field of the study and academic major of the researchers. Mehmet Doganay (MD) supervised all steps. The clinical trials were included if their intervention were by intravenous infusions of stem cells tested on the previously healthy persons newly suffering from sepsis or septic shock (Table 2). However, all phase I trials, independent of types of the underlying disease, so-called "condition," were reviewed for untoward side effects and safety of stem cell infusions. The clinical studies and review papers were included if they were released in the last ten years’ collection to prepare the manuscript.
| Study Title | Author or Researcher | Register Number | Country | Clinical Setting | Eligible Ages | Cell Type | Trial Phase | Dose and Route | Start Date | Finish Date | Reference | Pros and Cons |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellular immunotherapy for Septic shock: A phase I trial (CISS) | McIntyre et al. (7), 2018 | NCT02421484 | Canada | Septic Shock | 18 years and older | Allogeneic mesenchymal stromal cells | Phase I | 0.3 × 106/kg, 1.0 × 106/kg, and 3.0 × 106/kg-intravenous | 2015 | 2016 | 78 | The infusion of MSC’s septic shock seems safe. |
| Cellular immunotherapy for septic shock (CISS2) | McIntyre et al., 2019 | NCT03369275 | Canada | Septic shock | 18 years and older | Human Mesenchymal Stromal Cells | Phase II | Infusion of 300 million Cells Intravenous | 2017 | Not announced yet | 80 | The infusion of MSCs in septic shock seems safe. |
| The Results of the Russian Clinical Trial of Mesenchymal Stromal Cells (MSCs) in Severe Neutropenic Patients (pts) with Septic Shock (SS) (RUMCESS trial) | Galstian et al. (8), 2015 | NCT01849237 | Russia | Septic shock | 17 years and older | Mesenchymal stromal cells | Phase I/II | MSCs infusion of 1-2× 106/kg/day- intravenous | 2011 | 2012 | 81 | IV infusion of MSCs improved outcomes for severe sepsis |
| Effects of administration of mesenchymal stem cells on organ failure during the septic shock (CSM choc) | Gibot et al., 2016 | NCT02883803 | France | Sepsis | 18 years and older | Mesenchymal cells | Phase 1 | 106/kg intravenous | 2016 | 2019 | - | Ongoing |
| Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials | Lalu et al. (9), 2012 | Systematic review and meta-analysis evaluating 36 clinical Trials | USA | Various Clinical Settings | Various ages | Mesenchymal Stromal Cells | Phase I | Various | 2011 | 2011 | 82 | MSC therapy appears safe. |
| Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis | Thompson et al. (10), 2020 | Systematic review and meta-analysis evaluating 55 clinical Trials | Canada | Various Clinical Settings | Various ages | Mesenchymal Stromal Cells | Phase I (Safety) | Various | 2020 | 2020 | 83 | MSC therapy continues to appear safe |
Ongoing and Results of Stem Cells Studies (Trials, Systematic Reviews, and Metanalysis); Pros and Cons MSC, Mesenchymal Stem Cells
| Terms | Search Resultsa | Entire Databaseb |
|---|---|---|
| Synonyms | ||
| Stem cell | 36 studies | 7,935 studies |
| Progenitor cell | Three studies | 971 studies |
| Cells stems | One study | Six studies |
| Blast cell | Not found | 79 studies |
| Cell progenitors | Not found | Eight studies |
| Mother cell | Not found | Three studies |
| Cell | 38 studies | 63,329 studies |
| Cellular | Three studies | 4,103 studies |
| Bile salt-stimulated lipase | Not found | Four studies |
| Cholesterol esterase | Not found | One study |
| Lysophospholipase | Not found | Two studies |
| Stem | 37 studies | 68,119 studies |
| Process | 33 studies | 61,534 studies |
| Processus | Not found | 28 studies |
| Sepsis | 38 studies | 2,211 studies |
| Toxemia | Eight studies | 683 studies |
| Infection systemic | Not found | Three studies |
| Septicemia | Not found | 42 studies |
| Stem cell|sepsis | 38 studies | |
| Completed studies | 15 studies | |
| Filtered by sepsis (condition) | Six studies | |
| Interventional study by stem cell infusion | Five studies | |
| Age groups | ||
| Child (birth-17) | 14 studies | |
| Adult (18 - 64) | 38 studies | |
| Older adult (> 65) | 35 studies | |
| Study phase | ||
| Phase 1c | Five studies | |
| Phase 2d | Seven studies | |
| Phase 3d | Five studies | |
| Phase 4e | One study | |
| Not applicablee | Six studies | |
. Characteristics of Ongoing Clinical Trials of Stem Cells on Sepsis and Septic Shock Patients Searched in Clinicaltrials.gov Website Maintained by the National Library of Medicine (NLM)
2.3. Study Selection
After the inclusion of criteria, we selected 301 original articles (including 13 articles about animal phase I clinical trials and basic sciences research articles, 6 human phase II-III clinical trials, 12 systematic reviews, and meta-analysis, and 30 review articles), one WHO web page, and five clinical guidelines and experts consensus. One hundred fifty-two articles were not added to references to match the journal policy for a maximum of about 80 references for review articles. Lastly, MD selected eight references for designing context, ZBG included 27 references in the field of cell therapy in sepsis, GD included 17 references in the field of preclinical studies with stem cell therapy in sepsis model, and JS selected 31 references in the field of sepsis management, current problems, and human clinical trials.
3. Results
Stem cell therapy is a new concept. Most relevant articles belong to after 2010 and phase I trials. We have found about 148 clinical trials by “sepsis” and “stem cell” keywords (Clinicaltrials.gov Website) (accessed 8 May 2020). When the results were filtered by “completed”, the yield was 66. The results of seven of them were available. However, only one related to sepsis, two related to phase II trials, and no one related to the phase I and III trials. In searching the clinical trial website for the “Stem cell” and “Mesenchymal stem cells” (MSCs) keywords, we yield 80 studies among them, and when the search was filtered by “Completed”, yielded 29 studies. Only one study’s statutes were labeled as “results available”; however, its intervention was an unrelated drug.
3.1. Sepsis Management and Current Problems
Recent international consensus definitions for sepsis stated that “sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection” and septic shock is “a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone” (1).
From the first sepsis consensus in 1991 and its pediatric version in 2005 (11), many papers were published to update and increase the sensitivity and specificity of the definitions and supporting guidelines with the best up-to-date quality of evidence (1). Nevertheless, the definition criteria for sepsis diagnosis and treatment have been the scene of many debates and a definition for systemic inflammatory response syndrome (SIRS), infection, severe sepsis, and septic shock (12).
The Infectious Diseases Society of America (IDSA) did not endorse the Sepsis-3 guidelines due to the lack of differentiation between suspicious and definitive diagnosis of sepsis and septic shock, rigid recommendation to initiate early antibiotic therapy for every suspected sepsis within one hour, management of presumed tunnel or exit infections, empirical Vs. targeted, and combination Vs. so-called multiple antibiotic therapies, especially against Gram-negative infections and duration of therapy for sepsis.
Despite an increasing understanding of the arena, existing therapeutic has not advanced much beyond the longstanding mainstays. Current sepsis management has relied on several modalities. The serum level of lactate has been added as a significant criterion to assess sepsis’s risk and severity (1, 13). Early antibiotic therapy and restoration of perfusion and source control are the mainstays of therapy. Vasopressors advocated for patients with a lactate level higher than four mmol/liter or systolic blood pressure less than 90 mmHg. Sepsis treatment remains a significant challenge after the failure of over 100 clinical trials (5, 6). Many systematic reviews and meta-analyses were published to evaluate sepsis treatment trials, almost all with negative results (14-18).
Several deviant trends in sepsis research proposed the causes of the failure of trials and reaching the goals of decreasing sepsis mortality. These included controlling the early hyperinflammatory stage, inhibiting single inflammatory mediators, and intervention timing (4, 19). Steroids are frequently used to control the early hyperinflammatory stage. It was a cause of many long debates in the clinical management of sepsis. Rochwerg et al. (16), in a recent systematic review and meta-analysis, calculated a small reduction in severe sepsis mortality in severe sepsis treated with a corticosteroid. Another recent Cochrane systematic review by Annane et al. (20) concluded that low-quality evidence indicates a decrease in mortality. However, other researchers challenged the studies’ results, and the authors requested to disclose the potential conflicts of interest (21). They argued that more subgroup analysis is needed, and it seems that the beneficial effect of corticosteroids is mostly hemodynamic than modulating the abnormal inflammatory response (19).
The low efficacy of available therapeutic modalities, along with the high mortality rate of sepsis and septic shock, pose a significant challenge, forcing researchers to seek new treatment options.
3.2. Cell Therapy in Sepsis
Advancements in the cell biology field have led to a better understanding of pathological mechanisms besides physiological development (22). Stem cells are divided into two main subgroups, which show significant different properties and are classified into adult and embryonic stem cells (ESCs) (23, 24). ESCs are derived from early preimplantation embryos and can differentiate into endoderm, mesoderm, and ectoderm cells. However, it may not be applied directly in clinical practice due to ethical concerns of ESCs (23). Kasuda et al. (25) reported that the injection of IPSs-derived hematopoietic embryoid bodies ameliorated the lungs’ condition and significantly decreased the mortality rate in an animal model study of septic peritonitis.
Researchers reported that the number of mobilized HSCs was increased in sepsis (26). Skirecki et al. (27) reported the usage of the increasing number of HSCs and their relationship between mortality as a novel biomarker in septic shock patients. The second leading group of adult stem cells consists of stromal or mesenchymal stem cells (MSCs), which is currently a stem cell therapy with widely-used clinical efficacy (28). MSCs can be found in virtually all adult tissues and fetal tissues (24, 29, 30). MSCs have reliable results based on their multipotent differentiation potential and immune regulatory function for regenerative medicine. The International Society for Cellular Therapy has reported the proposal of the defined criteria for the MSCs in 2006 (24).
MSCs are potential therapeutic agents that can play different roles in the pathophysiology of sepsis, anti-inflammation, coagulation, antimicrobial effect, immune modulation, and tissue repair (28). According to considerable studies in the literature, mortality is induced by sepsis-related to immunosuppression (31, 32). Sepsis leads to T cell dysfunction. Moreover, it affects the functionality of the monocytes, macrophages, and dendritic cells (33). As a result of the functional drawbacks of antigen-presenting cells and T cells, an entire depression of normal immune response occurs in sepsis (33). The “cytokine storm” in sepsis is appeased via the immune regulation properties of MSCs, which cause a decrease in inflammation. The mechanism of the effect of MSCs is related to the reduction of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), Interleukin 6 (IL-6), and Interferon-gamma (IFN-γ), with an adjunct enhancement in anti-inflammatory cytokines such as IL-10, transforming growth factor-β (TGF-β) and IL-13. Moreover, MSCs reduce immunoglobulin G (IgG) production from B cells (33-36). Many studies have demonstrated that MSCs showed their effect on IL-10-mediated mechanism in sepsis (37-39). Moreover, MSCs treatment significantly decreased bacterial colony units and bacterial proliferation, besides decreasing the number of bacteria in the blood and enhancing bacterial clearance (40, 41). The shreds of evidence show that human MSCs are skilled in inhibiting nuclear factor-κB (NF-κB) activation, a pathway of the microorganisms-induced inflammatory response, thereby reducing organ damage (42).
Nowadays, the use of exosomes to target specific cells is also rising cellular components. Exosomes are small extracellular vesicles with size ranges around 30 - 150 nm derived from several cells, such as MSCs, crucial for intercellular communication (43). Several studies showed MSC-derived exosomes facilitated the anti-inflammatory response; however, only limited reports directly studying the effects of exosomes in sepsis (43-45). Wang et al. (44) reported that microRNA-223 (miR-223) knockout MSC-derived exosomes exacerbated the tissue injury in sepsis, while miR-223 showed protective and anti-inflammatory effects.
Chimeric antigen receptor (CAR) T cell therapies have been a novel cellular therapy tool in sepsis. CAR T cells are linked to T cell activation and cytokine secretion, leading to a specific antigen to destroy target cells without the need for antigen presentation by antigen-presenting cells. CAR T cell therapies in sepsis mainly depend on the encouraging outcomes from the study of Aspergillus fumigatus fungus elimination by Dectin-1 modified cytotoxic T cells (46). Cell therapies, either stem cells or T cell therapies, could intervene at a sepsis’s pathophysiology level.
3.3. Preclinical Studies with Stem Cell Therapy in Sepsis Model
Animal sepsis models are mandatory for a better understanding of sepsis development mechanism and determining treatment options. Various animal models have been developed to examine the pathogenesis of sepsis and create reproducible systems for testing new therapeutic agents (47-49). The selection of animal species to be used in the study depends on many factors. Some small laboratory animals are generally used in sepsis models, such as mice, rats, guinea pigs, and rabbits. These animals are suitable for experimental protocols and easy to obtain, maintain and reproduce due to their relatively low cost, short generation time, the presence of transgenic species, relative ease of housing and care, and easier application methods (47, 50, 51).
The experimental murine models of sepsis generally fall into two main categories: non-surgical and surgical models (47, 52-54). Multidrug-resistant (MDR) pathogens have been implicated in infections in healthcare settings over the past few decades (especially K. pneumoniae, A. baumannii, E. coli) leading to causative microorganisms of hospital-based infections like sepsis and septic shock. Antimicrobial treatment of MDR infections has become increasingly difficult due to the limited treatment options available and the absence of a new antibiotic choice; alternative treatment options have emerged (55). Therefore, stem cell therapy is also considered a treatment option for sepsis with MDR pathogens. Mesenchymal stem cells have shown hope in experimental murine models as they reduced mortality and bacteremia in sepsis (35). Gonzalez-Rey et al. (35) used human and mice adipose-derived MSCs intraperitoneally for sepsis induced by CLP in Balb-c mice, and they reported that MSCs significantly improved the severity of colitis, weight loss, diarrhea and inflammation, and increasing survival. Bi et al. (56) developed sepsis with CLP in C57BL/6 mice and injected bone marrow allogeneic stem cells into mice. The therapeutic benefits are reported by increased prevention from body loss, survival rate, and inflammatory response suppression (40). Hall et al. (40) investigated the effect of bone marrow-originated MSCs in polymicrobial sepsis induced by CLP in Balb-c mice, and they demonstrated that MSCs increased the ability of neutrophils to phagocytize bacteria and to promote bacterial clearance (57). Kim et al. (57) induced toxic shock syndrome with staphylococcal enterotoxin B (SEB) in C57BL/6 mouse and used bone marrow MSCs for modulating the host-derived proinflammatory response. They reported that MSCs suppressed proinflammatory cytokines, but they are insufficient to raise survival. Dinc et al. (58) developed sepsis with carbapenem-resistant K. pneumoniae in neutropenic mice and reported that MSCs give an advantage with combined therapy with colistin in sepsis treatment. Similarly, other studies investigated the therapeutic potential of MSCs in different sepsis models of Balb-c, NOD SCID, or C57BL/6 mice, and they demonstrated that MSCs could be an effective treatment option for sepsis (59-61).
3.4. Clinical Trials with Stem Cells
MSCs are considered the best potential candidate for this purpose due to their distinctive advantages among different stem cells. These include immunomodulatory and antimicrobial effects, the ability to protect against organ failure, and modulate inflammatory cascades of sepsis and septic shock (62, 63).
A phase I trial of allogeneic freshly cultured bone marrow-derived MSCs enrolled nine sepsis patients and 21 control cases (7). The researchers infused doses up to 250 million cells and found them safe. No adverse events were reported. Moreover, there were no significant differences in cytokine levels between interventional and observational groups. In another study, a phase I/II randomized controlled trial, the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure was reported safe and efficient (64).
A recent phase I dose-escalation safety trial of septic shock patients compared 49 cytokines and biomarkers levels in the groups of healthy and septic shock participants (65). The trial used allogeneic bone marrow-derived mesenchymal in various doses (0.3, 1.0, or 3.0 million mesenchymal stem/stromal cells/kg body weight stem/stromal cells). The researchers concluded that the cells modulated the innate immunity and cytokines consistent with a safe response.
The Russian trial of MSCs on neutropenic patients with septic shock enrolled 27 patients aged 33 - 81 years, median 55 years in 2015. All patients were under chemotherapy because of various malignancies. They were randomly assigned to conventional therapy and those with additive MSCs treatment; A dose of 106/kg of MSCs was administered intravenously 10 hours after the diagnosis of septic shock. After 28 days of follow-up, there was a minimal decrease in short-term mortality in the treated group with MSCs. However, no change was observed in terms of organ failure and long-term survival (8).
A systematic review and meta-analysis of clinical trials was done in 2011 by Lalu et al. (9). They searched all clinical trials that investigated therapy safety with mesenchymal stromal cells (9). A total of 36 clinical trials in various clinical conditions, with 1,012 patients were selected in adults and children. They found no association between mesenchymal stromal cell therapy with toxicity after infusion, organ failure, infection, death, or malignancy. Overall, therapy with mesenchymal stromal cells was evaluated as a safe therapy (9).
Another systematic review and meta-analysis of clinical trials for the safety of mesenchymal stromal cells was conducted in 2020 by Thompson et al. (10) Fifty-five clinical trials that met the inclusion criteria were analyzed, involving 2,696 patients. The trials found a small increase in fever episodes following the infusion of MSCs. However, no toxicity episodes were reported due to infusion, thrombosis, or emboli phenomenon, malignancy, or death. The authors concluded that there are enough documents for the safety of MSCs therapy, and the researcher can now enter the phase II trials to measure the efficacy of these types of stem cells in the treatment of sepsis and septic shock (10).
4. Conclusions
Although an achievement in the pathogenesis, diagnosis, intensive care improvement, and new treatment modalities, sepsis, and septic shock are still grave health problems worldwide with untoward sequala, high mortality, and health cost. Antibiotic resistance is also an increasing problem in ICUs and hospitals. Cell therapy is a promising treatment option for sepsis. Indeed, immunomodulatory properties, antimicrobial activity, and the capacity to protect against organ failure enhance tissue injury resolution, tissue repair, and restoration after sepsis confer MSCs with a significant advantage to treat the immune and inflammatory dysfunctions associated with sepsis and septic shock. Although early clinical trials seem promising and have beneficial effects, we need more controlled clinical studies, especially in phases II and III.