1. Background
In December 2019, coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, and spread rapidly around the world (1). This pandemic was caused by a novel betacoronavirus, known as severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). Its genome is partially similar to other coronaviruses, such as SARS-CoV and the Middle East respiratory syndrome-coronavirus (MERS-CoV). SARS-CoV-2 has a high reproductive number with a long incubation period and a lower fatality rate (significantly higher in high-risk patients with comorbidities) as compared to SARS-CoV and MERS-CoV (2).
The main features of COVID-19 include diffuse alveolar involvement and respiratory failure. Although renal involvement has not been reported as the first presentation of this disease, nephrologic problems occur, especially in patients admitted to the intensive care units (ICUs). Since the RNA of coronavirus has been detected in the plasma of 15% of patients by real-time-polymerase chain reaction (RT-PCR) (3), it seems that it can penetrate into other solid organs, such as the kidneys. In a previous report, hypoxic respiratory failure and oliguric renal failure after open surgery for aortic dissection were reported in a patient who died of COVID-19, and the pathological report revealed evidence of direct renal infection with SARS-CoV-2. Also, electron microscopy of proximal tubules showed vacuolization, degenerated epithelial cells with multiple intracellular viral clusters, and intracytoplasmic viral arrays within tubular epithelial cells. This report suggested that direct infection of the renal tissue by SARS-CoV-2 occurs in the setting of AKI in COVID-19 patients (4).
In another study, acute kidney injury (AKI) was reported in 6.7% of patients infected with COVID-19, and the mortality rate of patients with AKI was 91.7% (5). Also, a study by Cheng indicated a mortality rate of 5.1% for AKI in patients with COVID-19, and indicators of renal involvement were associated with a higher risk of mortality. In this cohort, 16.1% of the patients expired at the hospital (6). Moreover, Zhao et al. (7) reported AKI in 5.5% of adult patients with COVID-19 (7). On the other hand, Wang et al. reported an increase in the serum creatinine level in 10.8% of patients, none of whom met the diagnostic criteria for AKI (8).
In the context of COVID-19, the results vary between adults and children. In a study by Lu and colleagues at Wuhan Children’s Hospital among 1391 children, who were tested for COVID-19, only one ten-month-old infant died with multiple organ failure, whereas no major organ involvement was found in the rest of the patients (9). Overall, information about renal and urinary tract involvement in patients with COVID-19 is limited, and further investigations are needed. Also, patients with chronic renal diseases, and those who undergo dialysis are susceptible to this novel viral infection and its complications (10).
2. Objectives
There is not adequate information about renal and urinary tract involvement in children infected with COVID-19. Therefore, in this study, we aimed to determine the prevalence of renal and urinary tract disorders in pediatric patients with COVID-19 admitted to Mofid Children’s Hospital, which is a referral hospital for pediatric patients with COVID-19 in Iran.
3. Methods
This cross-sectional study was conducted on 71 pediatric patients with COVID-19, admitted to the Tertiary Children’s Hospital of Shahid Beheshti University of Medical Sciences in Tehran, Iran. All consecutive patients with a diagnosis of COVID-19, who were admitted to Mofid Children’s Hospital from February 19 to April 19, 2020, were enrolled in this cohort study. Diagnosis of COVID-19 was established, according to the guidelines provided by the Iranian Ministry of Health. The diagnostic criteria, based on the Iranian Expert’s Consensus Statement, were as follows (11):
Clinical Data: Symptoms including fever, dry cough, and chills, or severe hypoxemia, acute hypercapnia, hemodynamic instability, and decreased level of consciousness in the absence of fever.
Laboratory Data: High levels of C-reactive protein (CRP), thrombocytopenia, or lymphocytopenia.
Imaging Data: Pulmonary involvement in favor of COVID-19 on chest X-ray (CXR) or computed tomography (CT) scan.
Positive results on high-throughput sequencing or RT-PCR.
According to the abovementioned criteria, the patients were divided into three groups, as follows (11):
Definite Cases: Children with a history, signs, and symptoms of COVID-19, together with abnormal chest CT scan results and positive PCR test results.
Suspected Cases: Children with a history, signs, and symptoms of COVID-19, abnormal chest CT scan results suggesting COVID-19 (if other causes of abnormal CT are ruled out), and negative PCR results.
Rejected Cases: Children with negative PCR results and abnormal chest CT scan due to causes, except COVID-19.
The patients’ demographic characteristics, clinical symptoms (blood pressure, heart rate, respiratory rate, and body volume indices), laboratory data (complete blood count, urinalysis, liver function tests, blood urea nitrogen, first and serial serum creatinine levels, biochemical tests, erythrocyte sedimentation rate [ESR], CRP, and lactate dehydrogenase [LDH]), RT-PCR results for COVID-19, kidney ultrasonography, and medications were extracted from the electronic medical records. The glomerular filtration rate (GFR) was also calculated based on the Schwartz formula (12). Also, a normal serum creatinine level was considered as the local reference (0.5 to 1.0 mg/dL for children ages 3 to 18 years and 0.3 to 0.7 mg/dL for children younger than age 3) (13). AKI was also defined based on the pediatric risk, injury, failure, loss of kidney function, and end-stage kidney disease (pRIFLE) criteria (Table 1) (14).
| pRIFLE Stage | eCCl | Urine Output |
|---|---|---|
| R = Risk of renal dysfunction | decreased by 25% | < 0.5 mL/kg per hour for 8 hours |
| I = Injury to the kidney | decreased by 50% | < 0.5 mL/kg per hour for 16 hours |
| F = Failure of kidney function | decreased by 75% | < 0.3 mL/kg per hour for 24 hours or anuria for 12 hours |
| L = Loss of kidney function | Persistent failure > 4 weeks | |
| E = End-stage renal disease | Persistent failure > 3 months |
The pRIFLE Classification for AKI (14)
The baseline serum creatinine level was defined as the initial serum creatinine level upon admission. Also, the onset time of AKI was defined as the earliest time of serum creatinine change meeting the RIFLE criteria.
The research protocol and informed consent were approved by the ethics committee of Pediatric Nephrology Research Center and Research Institute for Children’s Health of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3.1. Statistical Analysis
Data were reported as mean ± SD, and categorical variables were expressed as percentages. P-value less than 0.05 was considered statistically significant.
4. Results
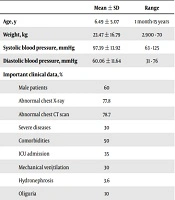
A total of 71 pediatric patients with COVID-19 were included in this study. The result of COVID-19 PCR was positive in 40.9% of the patients. The rest of the children were diagnosed based on the criteria mentioned above. The mean age of the patients was 6.49 ± 5.07 years (range: 1 month-15 years), and 60% of them were male. Also, the mean weight of the patients was 23.4 ± 16.7 kg (range: 2.9 - 70 kg). The clinical features and laboratory data of the subjects are presented in Tables 2 and 3.
| Mean ± SD | Range | |
|---|---|---|
| Age, y | 6.49 ± 5.07 | 1 month-15 years |
| Weight, kg | 23.47 ± 16.79 | 2.900 - 70 |
| Systolic blood pressure, mmHg | 97.39 ± 13.92 | 63 - 125 |
| Diastolic blood pressure, mmHg | 60.06 ± 11.64 | 31 - 76 |
| Important clinical data, % | ||
| Male patients | 60 | |
| Abnormal chest X-ray | 77.8 | |
| Abnormal chest CT scan | 78.7 | |
| Severe diseases | 30 | |
| Comorbidities | 50 | |
| ICU admission | 35 | |
| Mechanical ven\tilation | 30 | |
| Hydronephrosis | 3.6 | |
| Oliguria | 10 | |
| Edema | 7.7 | |
| AKI | 34.5 | |
| Stage I (risk group) | 20 (of AKI group) | |
| Stage II (injury group) | 25 (of AKI group) | |
| Stage III (failure group) | 55 (of AKI group) | |
| Need for dialysis | 0 | |
| In-hospital death | 12.67 |
Clinical and Demographic Data of the Patients
| Mean ± SD | Range | |
|---|---|---|
| Leukocyte count, × 10⁹/L | 9409.66 ± 4701.65 | 2000 - 25,000 |
| Lymphocytes, % | 40.03 ± 17.2 | 14 - 75 |
| Platelet count, × 10⁹/L | 248529.4 ± 157790.2 | 60,000 - 793,000 |
| Hemoglobin, g/dL | 11.13 ± 2.32 | 5.50 - 18.30 |
| ESR, mm/h | 43.66 ± 11.05 | 31 - 60 |
| CRP, mg/L | 28.52 ± 24 | 4 - 112 |
| LDH, U/L | 668.93 ± 378.94 | 135 - 2300 |
| Serum sodium, mEq/L | 137.06 ± 4.19 | 130 - 155 |
| Serum potassium, mEq/L | 4.13 ± 0.61 | 2.90 - 5.80 |
| Serum calcium, mg/dL | 8.4 ± 1.26 | 4.1 - 10.1 |
| Serum phosphate, mg/dL | 4.24 ± 1.43 | 2 - 8 |
| Serum magnesium, mg/dL | 2.12 ± 0.24 | 1.7 - 2.9 |
| PCO2, mmHg | 29.85 ± 20.17 | 20 - 87 |
| Serum bicarbonate, mEq/L | 17.70 ± 12.49 | 3 - 47 |
| Blood urea nitrogen, mg/dL | 15.08 ± 10 | 9 - 90 |
| Serum creatinine, mg/dL | 1.30 ± 1. | 0.4 - 9.6 |
The Laboratory Data of the Patients
On admission, 10% of the patients had oliguria, 7.7% had edema, and 3% had hypertension. No patients showed signs of hypotension. The first urinalysis showed proteinuria, leukocyturia, and hematuria in 46%, 24%, and 23% of the patients, respectively. Overall, 40.7% of the patients showed some degree of renal involvement. During hospitalization, the peak serum creatinine level was 9.6 mg/dL (range: 0.4 - 9.6 mg/dL; mean: 1.3 ± 1 mg/dL), and the prevalence of AKI was 34.5%. Based on the pRIFLE classification, stage I (risk group) was found in 20% of the patients, stage II (injury group) in 25% of the patients, and stage III (failure group) in 55% of the patients with AKI.
Compared to patients with normal renal function, those with elevated serum creatinine levels on admission were predominantly older and more severely ill. The total mortality rate of the patients was 12.67%. The rate of in-hospital death among patients with AKI was estimated at 30%. The incidence of ICU admission and respiratory therapy in patients with elevated baseline serum creatinine levels was significantly higher than those with normal serum creatinine levels upon admission (35% vs. 16.1% and 30% vs. 15.6%, respectively). The final laboratory data showed mild abnormalities in the serum creatinine level of 30% of the patients, and the mean final level of serum creatinine was 0.83 ± 0.8 mg/dL (range: 0.3 - 4.8 mg/dL). The final urinalysis revealed leukocyturia in 12.5% of the patients, hematuria in 25% of the patients, and proteinuria in 33.3% of the patients.
5. Discussion
The results of this prospective study, which was conducted in a tertiary teaching hospital in Tehran, Iran, revealed the high prevalence of renal involvement in hospitalized pediatric patients with COVID-19. In this study, more than 40% of the patients showed evidence of renal involvement, with elevated serum creatinine levels in more than 34.5% of these patients. This is the first study examining the prevalence of renal and urinary tract involvement in pediatric patients with COVID-19 in Iran. The presence of renal involvement was associated with a higher in-hospital mortality rate. Compared to patients who did not develop AKI after admission, the patients who developed AKI had a more severe disease and even showed failure of other organs, besides a higher in-hospital mortality rate. In comparison, patients with acute renal failure were more likely to be admitted to pediatric ICUs. They were also more likely to be managed with vasopressors for hypotension, have multiple organ failure, and receive ventilation therapy during hospitalization.
In this regard, Cheng et al. (6) assessed the relationship between markers of renal insufficiency and death in patients with COVID-19 in a cohort study of 701 individuals in December 2019 in Wuhan, Hubei Province, China. The results showed that 16.1% of the patients expired at the hospital. On admission, proteinuria was detected in 43.9% of the patients, hematuria in 26.7% of the patients, and increased serum creatinine levels in 14.4% of the patients. The rate of AKI among patients was 5.1% during admission. Patients with elevated baseline levels of serum creatinine showed a significantly higher incidence of AKI as compared to patients with a normal baseline serum creatinine (11.9% vs. 4.0%) (6).
On the other hand, the involvement of multiple organs, including the liver, kidneys, urinary tract, and gastrointestinal tract, has been reported in COVID-19 patients (4). In a study by Zhao et al. (7), comorbidities, including kidney disease, hypertension, chronic lung disease, malignancies, and diabetes, were more common in the severe disease group (40% vs. 14.8%; P = 0.009). They also reported acute pulmonary, renal, and cardiovascular involvement in the patients, and the rate of AKI was 5.5% in this report (15). Surprisingly, in New York City, USA, the rate of AKI was estimated at 36%, according to the kidney disease: Improving global outcomes (KDIGO) definition, 14.3% of whom required renal replacement therapy (RRT). Also, most of the AKI cases (89.7%) and 96.8% of the patients who required RRT were on mechanical ventilation. In this report, a mortality rate of 35% was measured in patients with AKI (16). Overall, AKI is strongly associated with increased mortality and morbidity rates in severely ill patients (17-19).
We found that patients with AKI were more likely to be admitted to ICUs and receive respiratory support; therefore, renal involvement represented a higher risk of deterioration in these patients. In our study, indicators of renal involvement at admission and the increased level of creatinine were associated with a higher risk of mortality. Therefore, monitoring of renal function and urinalysis must be emphasized, even in patients with mild symptoms. Also, attention must be paid to altered kidney function test results and electrolyte imbalances after the hospitalization of pediatric patients with COVID-19. Early detection of renal failure and timely management, involving appropriate hydration, adequate hemodynamic, electrolyte, and acid base support, and improved protection of the kidneys with avoidance of nephrotoxic drugs, may help improve the prognosis of these patients.
The etiology of nephrologic problems in patients with COVID-19 is likely to be multifactorial. This novel virus may exert direct effects on the renal tissues of infected patients (3). Recently, it has been reported that angiotensin converting enzyme 2 (ACE2) is a cell entry receptor for COVID-19 (20). In this regard, Li et al. (21) in a human study revealed that ACE2 expression in the renal tissue was nearly 100 folds higher than in the lungs. Accordingly, renal involvement may be caused by coronavirus through an ACE2-dependent pathway.
Another etiological factor for renal involvement in COVID-19 is the deposition of immune complexes, such as viral antigens and antibodies or virus-specific T-cell lymphocytes or antibodies in the kidney cells. Besides, infection-induced cytokines may have indirect effects on the renal tissue and other organs due to hypoxia, hypoxemia, and shock. Further research is needed to determine the potential kidney pathophysiology in patients with COVID-19. The first reports clarified that COVID-19 infection and mortality are rare in the pediatric population. However, recent clinical and epidemiological evidence shows that no particular age group is immune to this viral infection. Renal involvement is frequent in severe pediatric cases and usually presents as AKI in the context of multiple organ failure, caused by direct viral invasion or acute inflammatory responses (22).
Pediatric patients with COVID-19 are susceptible to AKI, which can increase the mortality rate. On the other hand, pediatric patients with chronic kidney diseases and other chronic disorders are susceptible to severe COVID-19, which can increase the total rate of mortality in this group. In children, COVID-19 may be less severe as compared to adults (23, 24). In this regard, Bush et al. (25) reported the case of a 13-year-old male from USA, who acquired COVID-19 infection five years post-kidney transplantation, with excellent clinical outcomes in a very short-term follow-up. Also, Melgosa et al. (26) reported 16 pediatric patients with chronic renal involvement who were diagnosed with COVID-19 in Spain. The severity of symptoms was mild in most patients with limited radiological findings (26).
Moreover, according to a study by Roberton et al. (27), there are additional reports of neonatal, maternal, and under-five mortality, resulting from disruptions in the delivery and utilization of health services and decreased access to food. Therefore, pediatricians need to be aware of the risks of these problems and should carefully evaluate all pediatric patients with chronic diseases in outpatient clinics for early detection of COVID-19 and prevention of multiple organ failure, resulting in death. It is certain that timely supportive therapy and accurate patient control are useful methods for the management of this group of patients (28).
The present study has several limitations. First, we did not have access to the baseline laboratory data, and the baseline BUN and serum creatinine levels of many patients were not available, which might have led to the misdiagnosis or underestimation of AKI. Second, although we tried to adjust for many confounders, other unknown confounders might have played a role. Third, because of the outbreak, the clinical and paraclinical data of our patients were not available after discharge; therefore, we could not demonstrate the effects of the virus on the outcomes and long-term complications. Therefore, further investigations are required to determine the precise effect of this virus on long-term kidney function and the prevalence of CKD in pediatric patients. In future studies, we aim to follow-up these patients for several months and will report the long-term renal complications of COVID-19 in these children.
5.1. Conclusions
The prevalence of AKI in pediatric patients infected with COVID-19 was high in our tertiary hospital. The presence of AKI in hospitalized pediatric patients was associated with an increased risk of mortality. Therefore, pediatricians should be aware of renal involvement in patients with COVID-19 infection. Also, early detection of renal involvement, based on the clinical and paraclinical parameters, besides effective management, may help reduce the mortality rate of patients with COVID-19.