1. Background
Acute lower respiratory infection (ALRI) is one of the main causes of morbidity and mortality in children under five years of age (1). The respiratory syncytial virus (RSV) remains its leading etiological factor (2, 3), identified over 50 years ago as an RNA virus of the family Pneumoviridae, genus Orthopneumovirus (4, 5). Although RSV infections occur in all age groups, the most severe course is observed among children. Almost every child by the age of two experiences the infection, with the peak incidence in two and three months of age. This period corresponds to the nadir concentration of maternal IgG (2). The clinical manifestations include both mild upper respiratory infections and severe infections of the lower tract, such as bronchiolitis and pneumonia. The two latter ones can lead to hospitalization and severe complications, including respiratory failure (6).
Such a significant clinical problem requires a search for both effective methods of treatment and prevention of the infection. Although several vaccines are at various stages of clinical trials, no fully safe and effective licensed product has been developed so far (7). The only widespread prophylactic method of severe ALRI caused by RSV in premature infants remains palivizumab, a specific monoclonal antibody directed against an epitope of the F glycoprotein of the virus (8). Besides preventive and therapeutic methods, risk factors for severe RSV infections in children are also investigated to define the groups of patients who may require special treatments and experience complications.
2. Objectives
The study aimed to evaluate the manifestations of RSV infection in hospitalized children younger than 18 months of age and predictors of disease severity, as well as their comparison with the same age group hospitalized due to ALRI of different etiology.
3. Methods
3.1. Material
In this retrospective study, medical records were analyzed in 448 children (179 girls and 269 boys) under the age of 18 months hospitalized due to ALRI in the Department of Pediatrics, Nephrology, and Allergology of the Military Institute of Medicine in Warsaw between 1/01/2018 and 1/03/2020.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria were (1) Clinical symptoms of ALRI confirmed by imaging study and (2) Age under 18 months. The exclusion criteria were (1) Primary immunodeficiency; (2) chronic lung disease; (3) congenital heart defect; (4) cardiovascular, liver, kidney, endocrine, hematological, neurological, gastroenterological, and other diseases that could contribute to immunodeficiency; (5) immunosuppressive therapy; and (6) Palivizumab administration.
3.3. Definitions and Diagnostic Criteria
The diagnosis of ALRI was based on the clinical examination following the applicable recommendations (9). Acute bronchitis was diagnosed based on the presence of cough lasting less than three weeks, which may be accompanied by wheezes and rhonchi (9-11). The additional symptoms suggestive of pneumonia were fever above 38°C, tachypnea, and intercostal retractions, combined with the crackles as the most indicative respiratory sound (9). Each clinical suspicion of ALRI was confirmed by chest X-ray or lung ultrasound. The radiographic features of pneumonia were consolidation, perihilar peribronchial thickening, and/or interstitial infiltrates, possibly accompanied by atelectasis (9, 12). The ultrasound features of pneumonia included subpleural consolidations and B-line artifacts (13).
Bronchiolitis was defined as an acute inflammatory process within the bronchioles characterized by acute-onset respiratory symptoms in children younger than two years of age, preceded by a viral infection of the upper respiratory tract and expiratory dyspnoea developing with the presence of wheezing and/or rales (14, 15). Respiratory failure, as a possible complication of ALRI, was defined by hypoxemia (an arterial oxygen tension (PaO2) of < 60 mmHg) with or without associated hypercapnia (an arterial carbon dioxide tension (PaCO2) of > 45 mmHg) (16).
3.4. Division of the Subgroups
The analysis was performed on the total study group, as well as subgroups of children with positive (n = 130; 29.02%) and negative (n = 318; 70.98%) results of the nasal swab for RSV detection. The rapid-VIDITEST RSV test, a one-step colored immunochromatographic test, was used for the detection of RSV antigens in the nasal discharge of all children qualified for the study. During the test, a sample collected from the nasopharynx with a swab was reacted with anti-RSV antibody-coated particles on the strip test. In the case of a positive result, the specific antibodies reacted with the conjugate and formed a colored line. The test sensitivity was 95%, and specificity was > 99% (17).
Three age groups were identified in the study population, as follows: Children in their first six months of life (n = 256; 57.14%), children between the sixth and 12th months of life (n = 90; 20.09%), and children from their 12th months of life onwards (n = 102; 22.77%).
3.5. Clinical and Laboratory Data
In each group, the following data were analyzed: Manifestations of the disease, the length of hospitalization, treatment (oxygen therapy, antibiotics, inhaled and systematic glucocorticosteroids), and incidence of complications. Moreover, the possible risk factors and predictors of particular manifestations and the severity of the disease were analyzed, as follows:
1. Clinical Data: Age, birth weight, gestational age at birth, possession of older siblings, and family history of allergies (asthma, atopic dermatitis, or allergic rhinitis among parents and siblings);
2. Body temperature, blood oxygen saturation, heart rate, and respiratory symptoms on hospital admission;
3. Laboratory Results on Admission: White blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), level of hemoglobin (Hb), and capillary blood gases.
Imaging tests, including chest X-ray and lung ultrasound, were also evaluated to confirm the diagnosis of ALRI.
3.6. Statistical Analysis
The measures of central tendency and their spread, as well as statistical distribution, were determined for analyzed variables. The non-parametric Mann-Whitney U-test was used to compare the quantitative variables whose distribution was significantly different from the Gaussian distribution. The chi-square test was used for comparing qualitative data. The probability value of P < 0.05 was considered statistically significant. Microsoft Excel and Statistica 13 were used for analysis.
4. Results
4.1. Group Profile and Comparison
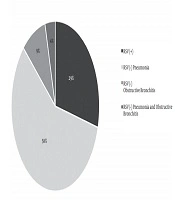
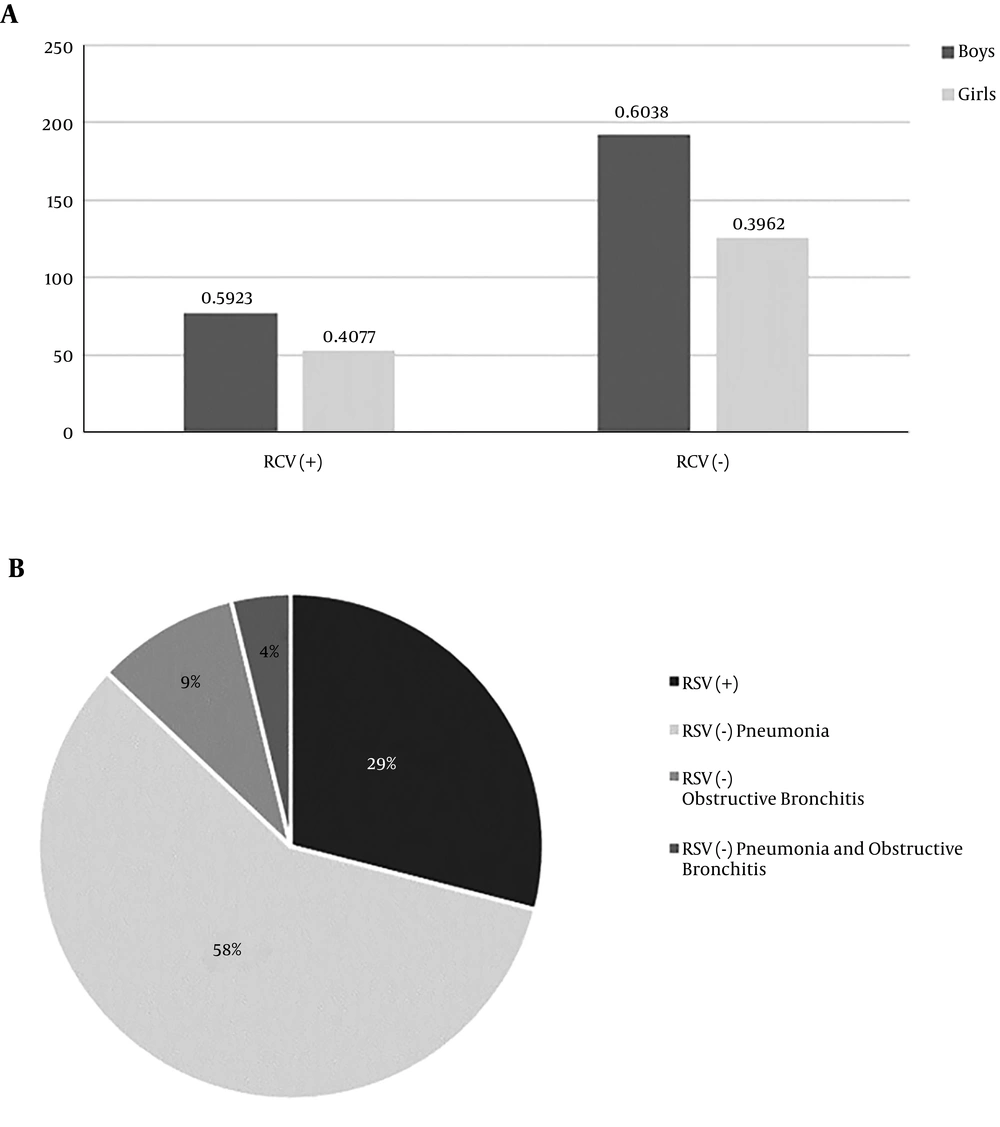
In the studied population, the most commonly diagnosed condition was pneumonia with an etiology other than RSV (n = 260; 58.04%), whereas RSV infection contributed to fewer than one-third of diagnoses (n = 130; 29,02%). In both RSV (-) and RSV (+) groups, the predominance of boys was observed (Figure 1). Children with RSV infection were younger. However, prematurity or low birth weight did not prevail in this group. The groups also did not differ significantly in terms of older sibling possession or positive family history of allergies (Table 1).
| Variable | Lower Quartile | Median | Upper Quartile | No. (%) | P Value |
|---|---|---|---|---|---|
| Age, mo | 0.001 | ||||
| RSV (+) | 2 | 3 | 7 | ||
| RSV (-) | 2 | 6 | 14 | ||
| Birth weight, g | 0.387 | ||||
| RSV (+) | 3112 | 3450 | 3770 | ||
| RSV (-) | 3122 | 3420 | 3757 | ||
| Hdba | 0.506 | ||||
| RSV (+) | 38 | 39 | 40 | ||
| RSV (-) | 38 | 39 | 40 | ||
| Presence of older siblings | 0.354 | ||||
| RSV (+) | 98 (75.38) | ||||
| RSV (-) | 226 (71.07) | ||||
| Family history of allergy | 0.743 | ||||
| RSV (+) | 24 (18.46) | ||||
| RSV (-) | 63 (19.81) |
aGestational age at birth.
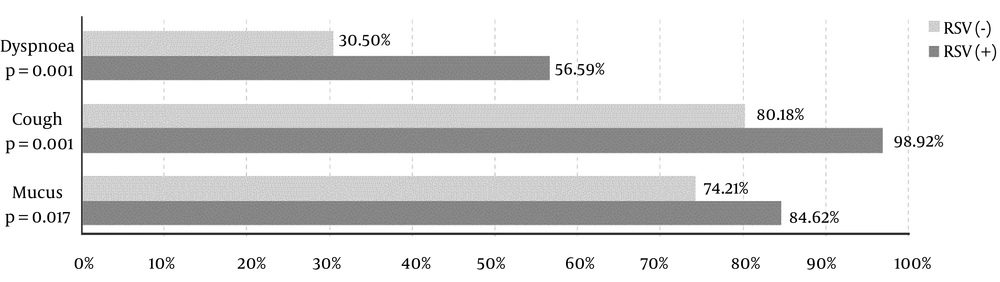
In the course of RSV infection, the lower inflammatory markers, lower NLR, and higher tendency to respiratory acidosis were noted. These children had significantly lower body temperature, lower blood oxygen saturation, and tachycardia. On hospital admission, they more often presented dyspnoea, cough, and discharge in the upper respiratory tract (Table 2 and Figure 2).
| Variable | Lower Quartile | Median | Upper Quartile | Association Type | Association | P Value |
|---|---|---|---|---|---|---|
| WBC, × 109/L | Negative | -3.35 | 0.001 | |||
| RSV (+) | 8.42 | 10.54 | 12.85 | |||
| RSV (-) | 9.12 | 11.96 | 15.28 | |||
| NLR | Negative | -2.93 | 0.003 | |||
| RSV (+) | 0.25 | 0.48 | 0.81 | |||
| RSV (-) | 0.31 | 0.66 | 1.34 | |||
| CRP, mg/dL | Negative | -2.28 | 0.023 | |||
| RSV (+) | 1.0 | 2.0 | 7.0 | |||
| RSV (-) | 1.0 | 4.0 | 15.0 | |||
| ESR, mm/h | Negative | -3.57 | 0.001 | |||
| RSV (+) | 8.00 | 14.00 | 26.00 | |||
| RSV (-) | 8.75 | 19.00 | 33.25 | |||
| HGB, g/L | N/A | -1.15 | 0.249 | |||
| RSV (+) | 108.0 | 112.5 | 120.0 | |||
| RSV (-) | 109.0 | 115.0 | 122.0 | |||
| pH | Negative | -2.17 | 0.030 | |||
| RSV (+) | 7.38 | 7.41 | 7.43 | |||
| RSV (-) | 7.40 | 7.43 | 7.44 | |||
| PCO2, mmHg | Positive | 2.28 | 0.022 | |||
| RSV (+) | 31.70 | 35.10 | 38.70 | |||
| RSV (-) | 30.50 | 32.90 | 35.85 | |||
| Temp | Negative | -3.80 | 0.001 | |||
| RSV (+) | 36.7 | 37 | 38 | |||
| RSV (-) | 36.8 | 37.5 | 38.9 | |||
| SpO2, % | Negative | -4.27 | 0.001 | |||
| RSV (+) | 94 | 96 | 98 | |||
| RSV (-) | 96 | 97 | 98 | |||
| HR | Negative | 2.47 | 0.014 | |||
| RSV (+) | 127 | 140 | 154 | |||
| RSV (-) | 120 | 134 | 148 |
Abbreviation: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HGB, hemoglobin; HR, heart rate; NLR, neutrophil-to-lymphocyte ratio; PCO2, partial pressure of carbon dioxide; RSV(-), RSV negative; RSV(+), RSV positive; SpO2, blood oxygen saturation; temp, body temperature; WBC, white blood cells.
The treatment used was different in both groups, which resulted from different etiologies of the infection. Oxygen therapy, inhaled glucocorticosteroids, and beta 2-adrenergic agonists were significantly more often used in children with RSV infection (P = 0.001). It should be noted that the last two methods of treatment were administered in this group only in the case of accompanying airway obstruction. Antibiotic therapy was used more often in the group with non-RSV infection (P = 0.001). Nevertheless, the hospitalization length was similar in both groups (median of five days).
4.2. Manifestations
In children with RSV infection, bronchiolitis (exclusively) affected 44 children (33.85%), whereas any additional manifestations (AOM, conjunctivitis, pneumonia, and respiratory failure) were observed in 86 children (66.15%). Pneumonia was the most common manifestation in children with both RSV-positive and negative infections. Respiratory failure was developed significantly more often in the RSV (+) group (Table 3).
| Variable | Values | P Value |
|---|---|---|
| AOM | 0.110 | |
| RSV (+) | 4 (3.08) | |
| RSV (-) | 15 (4.72) | |
| Bronchiolitis | N/A | |
| RSV (+) | 48 (36.92) | |
| RSV (-) | N/A | |
| Conjunctivi-tis | 0.138 | |
| RSV (+) | 4 (3.08) | |
| RSV (-) | 14 (4.40) | |
| DRF | 0.001 | |
| RSV (+) | 13 (10.00) | |
| RSV (-) | 6 (1.89) | |
| Pneumonia | 0.002 | |
| RSV (+) | 82 (63.08) | |
| RSV (-) | 260 (81.08) |
Abbreviation: AOM, acute otitis media; DRF, developing respiratory failure.
aValues are expressed as No. (%).
4.3. Predictors and Risk Factors of Particular Manifestations
Analyzing risk factors in the total group, we observed that AOM mainly affected children under the age of six months who had lowered inflammatory markers. On the other hand, conjunctivitis significantly more often affected children with a positive family history of allergies. In the group with RSV infection, pneumonia affected particularly children under the age of six months, with lower blood oxygen saturation and inflammatory markers, the features of acidosis, and fever-free course. However, no predictors of developing respiratory failure were noted. In the RSV (-) group, pneumonia was developed significantly more often in children with a positive family history of allergies. There was no association between manifestations and prematurity, low birth weight, or the presence of an older sibling at home in any group (Table 4).
| Age | WBC | NLR | CRP | Temp. | SatO2 | HR | pH | PCO2 | Dyspnea | Positive Allergic History | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Study Group | |||||||||||
| AOM | |||||||||||
| Association type | Negative | N/A | Negative | Negative | Negative | N/A | N/A | N/A | N/A | N/A | N/A |
| P value | 0.001 | 0.290 | 0.001 | 0.001 | 0.001 | 0.153 | 0.178 | 0.584 | 0.127 | 0.209 | 0.851 |
| Conjunctivitis | |||||||||||
| Association type | N/A | Negative | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Positive |
| P value | 0.276 | 0.040 | 0.151 | 0.215 | 0.810 | 0.947 | 0.383 | 0.426 | 0.941 | 0.209 | 0.037 |
| DRF | |||||||||||
| Association type | N/A | Negative | N/A | N/A | N/A | N/A | N/A | N/A | Positive | Positive | N/A |
| P value | 0.692 | 0.001 | 0.327 | 0.511 | 0.569 | 0.943 | 0.327 | 0.172 | 0.005 | 0.001 | 0.854 |
| Manifestation-overall | |||||||||||
| Association type | N/A | N/A | N/A | N/A | N/A | Negative | N/A | N/A | N/A | Positive | N/A |
| P value | 0.451 | 0.449 | 0.653 | 0.974 | 0.832 | 0.001 | 0.221 | 0.051 | 0.062 | 0.004 | 0.406 |
| RSV (-) | |||||||||||
| AOM | |||||||||||
| Association type | Negative | N/A | Negative | Negative | Negative | N/A | N/A | N/A | N/A | N/A | N/A |
| P value | 0.007 | 0.285 | 0.001 | 0.001 | 0.001 | 0.273 | 0.201 | 0.760 | 0.403 | 0.365 | 0.957 |
| Conjunctivitis | |||||||||||
| Association type | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Positive |
| P value | 0.079 | 0.301 | 0.762 | 0.519 | 0.519 | 0.826 | 0.976 | 0.203 | 0.736 | 0.451 | 0.047 |
| DRF | |||||||||||
| Association type | N/A | N/A | N/A | N/A | N/A | Negative | N/A | N/A | N/A | N/A | Positive |
| P value | 0.116 | 0.215 | 0.098 | 0.680 | 0.982 | 0.049 | 0.573 | 0.795 | 0.624 | 0.295 | 0.032 |
| Manifestation - overall | |||||||||||
| Association type | N/A | Negative | Negative | Negative | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| P value | 0.116 | 0.034 | 0.001 | 0.018 | 0.123 | 0.608 | 0.819 | 0.231 | 0.778 | 0.451 | 0.315 |
| RSV (+) | |||||||||||
| Pneumonia | |||||||||||
| Association type | Negative | Negative | Negative | N/A | Negative | Negative | Negative | Negative | Positive | Positive | N/A |
| P value | 0.008 | 0.006 | 0.016 | 0.063 | 0.044 | 0.001 | 0.048 | 0.039 | 0.022 | 0.001 | 0.316 |
| Conjunctivitis | |||||||||||
| Association type | N/A | Negative | Negative | N/A | N/A | N/A | Positive | N/A | N/A | N/A | Positive |
| P value | 0.170 | 0.033 | 0.019 | 0.077 | 0.104 | 0.182 | 0.028 | 0.208 | 0.590 | 0.850 | 0.001 |
| Manifestation-overall | |||||||||||
| Association type | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Positive | N/A |
| P value | 0.475 | 0.376 | 0.990 | 0.967 | 0.134 | 0.072 | 0.778 | 0.819 | 0.391 | 0.013 | 0.565 |
Abbreviation: AOM, acute otitis media; CRP, C-reactive protein; DRF, developing respiratory failure; HR, heart rate; NLR, neutrophil-to-lymphocyte ratio; PCO2, partial pressure of carbon dioxide; SatO2, blood oxygen saturation; temp., body temperature; WBC, white blood cells.
5. Discussion
According to global data, annually, about 34 million RSV-associated ALRI cases are reported in children under the age of five. Of them, more than three million children require hospitalization, and 66 to 199 thousand die each year (18). In this age group, RSV is a greater clinical problem than other common respiratory viruses, such as the influenza virus causing 28 to 111 thousand deaths per year or SARS-CoV-2 causing COVID-19 with childhood mortality close to 0% (19, 20).
In the temperate climate, most RSV infections occur between October and May, reaching a peak in January and February, which usually coincides with influenza seasons (4). Transmission of the infection occurs by the inoculation of nasopharyngeal mucosa or conjunctivas with secretions from the respiratory tract of an infected person. The incubation period ranges from two to eight days, whereas infectivity may persist up to three weeks, including asymptomatic people (2). It has been reported that the main source of virus transmission is school-going siblings who introduce it into the family unit. Both they and most household members undergo infection without any symptoms and only contribute to the infection of the most vulnerable infants and the elderly (21). In our study, however, we did not observe that having older siblings predisposes to greater susceptibility to RSV infection than to ALRI of different etiology in hospitalized children, as well as to a more severe course of the infection itself.
Children up to two years of age frequently experience a severe course of RSV infection other than the most common manifestation, bronchiolitis. Its occurrence among hospitalized patients ranges from 56% to 79%, which is consistent with our results (66.15%) (22-24). Differences appear, however, in the percentages of particular manifestations. In our study, the most common one was pneumonia (n = 82; 63.08%), reaching a much higher percentage than in previous studies. Wrotek et al. (22) reported that pneumonia occurred in 33% of patients under 22 months of age. Hall et al. (3) showed that this condition was developed in 51% of children aged 24 - 59 months, whereas according to Heikkinen et al. (24), only 3% of patients up to 13 years of age had such a manifestation (24). This difference seems to depend on the age of the study group, suggesting that pneumonia affects mainly the youngest children. Therefore, it can be concluded that in their case, chest X-ray or lung ultrasound on hospital admission is fully justified.
Both acute otitis media (n = 4; 3.08%) and conjunctivitis (n = 4; 3.08%) were surprisingly rare manifestations in our study group with RSV infection. According to the data in the literature, AOM affects 24% to even 58% of patients (22-26). However, these are mainly prospective studies, with the observation period up to one year after the end of hospitalization, which may lead to a higher percentage of this manifestation than in our retrospective study. Moreover, in the above-mentioned studies, only Wrotek et al. (22) described conjunctivitis as a complication, which affected 11% of their study group. Whereas in the study conducted by Souty et al. (27), regarding the occurrence of influenza-like viral infection symptoms, no statistically significant association was proved between the RS virus and conjunctivitis. In our study, we also did not observe that it occurred more often in RSV infection than in ALRI of different etiology. It is postulated, however, that the history of RSV infection in infancy may predispose to allergic rhinitis and conjunctivitis in the future (28, 29). It is associated with the penetration of the RS virus into the human body through conjunctivas, which interferes with cellular and humoral immunity, leading to chronic inflammation (28). Perhaps, this is also influenced by genetic predisposition, which would explain the more frequent occurrence of conjunctivitis in children with a positive family history of allergies.
In the literature, there are numerous studies regarding the risk factors of just RSV infection, as well as hospitalization in its course, including prematurity, low birth weight, exposure to tobacco smoke during pregnancy, no exclusive breastfeeding under the age of six months, and the presence of older siblings at home (3, 30). Yet, there are fewer studies that would define the risk groups for severe course of the infection on hospitalized children, which is the main subject of this study. Wrotek et al. (22) suggested that only the age above three months predisposed to AOM and pneumonia in the course of RSV infection. In our study, however, pneumonia was observed mainly in children younger than six months of age. However, Willson et al. (23) showed that infectious complications were developed mainly in children born before 35 weeks of gestation, indicating that most of the complications affected those without any significant risk factors. However, in our study, we could not prove that prematurity or low birth weight had an impact on the severity of infection in both RSV (+) and RSV (-) groups. We only found that lowered inflammatory markers on admission and clinical features, such as dyspnoea and lowered blood oxygen saturation, could herald the severe course of the disease and prolong hospitalization in children infected with the RS virus. None of these parameters, however, showed to be a predictor of developing respiratory failure.
The main limitation of our single-center, retrospective study was the small number of patients in the group diagnosed with RSV infection, as well as the disproportion in the size of both groups. Moreover, during the diagnosis of ALRI etiology, we only determined the presence of RSV infection without performing tests for the diagnosis of other viral or bacterial agents. Therefore, to draw unambiguous conclusions, further research is needed for prospective, multi-center types covering a larger group of patients.
5.1. Conclusions
1) The predictors of RSV infection among children with ALRI included younger age, lower inflammatory markers, lower NLR, a higher tendency to respiratory acidosis, worse vital signs, and increased clinical symptoms. However, prematurity or low birth weight did not increase the probability of this diagnosis in hospitalized children.
2) Pneumonia was the most common manifestation in children with both RSV positive and negative ALRI. In the group positive for the RS virus, it was a more common diagnosis than bronchiolitis.
3) The risk of severe disease in the course of RSV infection was greatest in children younger than six months of age who presented low inflammatory markers on hospital admission and had increased clinical symptoms. Nevertheless, widely known risk factors of RSV infection itself, such as prematurity or low birth weight, did not reflect the risk of developing pneumonia or respiratory failure in its course. Therefore, it seems advisable to perform the imaging of the lungs on admission and carefully monitor the child’s condition during hospitalization and follow-ups.
4) Special attention should be paid to children with ALRI, including those with non-RSV etiology, who have a positive family history of allergies, as they may be at a higher risk of the severe disease.