1. Context
The novel coronavirus, also known as Coronavirus 2019 (COVID-19), was first reported in Wuhan, Hubei province, China, in December 2019. It is a highly contagious disease, which wildly spread through respiratory droplets from infected individuals (1). In January 2020, the World Health Organization (WHO) declared a global public health emergency. According to the WHO reports, on March 8, 2020, COVID-19 was spread to 100 countries, and 100,000 cases were infected worldwide. Hence, on March 11, 2020, WHO declared COVID-19 a global pandemic (2).
Initially, the common symptoms of COVID-19 infection were fever, cough, and myalgia or fatigue. Less common symptoms included sputum production, headache, hemoptysis, and diarrhea (3). However, more severe symptoms may occur in the elderly, immunosuppressed patients, and those who suffer from some chronic diseases, including diabetes, cancer, and lung disease (3).
Since pregnancy is an immunosuppressive condition, pregnant women are at increased risk of developing viral infections, including COVID-19 (4). According to the evidence, the coronavirus family may cause poor obstetric outcomes (5-7), including miscarriage, fetal growth restriction, preterm labor, and maternal mortality (8, 9). Alfaraj et al. (2019) reported an incidence rate of 91% for neonatal adverse outcomes, such as intensive care unit admission, prematurity, and neonatal mortality (5).
Regarding the high potential of neonatal adverse outcomes, evidence on vertical transmission of COVID-19 during pregnancy and delivery are of crucial importance. However, evidence regarding the vertical transmission of COVID-19 during pregnancy and labor are not sufficient to conclude (9). Chen et al. (2020) evaluated the clinical records of 9 pregnant women with COVID-19 infection in a Wuhan University Hospital, China. They reported negative tests for COVID-19 from amniotic fluid, cord blood, neonatal throat, and breast milk samples in six patients (10). Li et al. (2020) published a case report about a woman with COVID-19 infection in her 35th weeks of gestation who delivered an infant via cesarean section. The infant was tested negative for COVID-19. They suggested that the virus is unlikely to be transmitted vertically (9). Wang et al. (2020) reported a pregnant case of positive COVID-19 infection who gave birth to an infant who was tested negative for Severe acute respiratory syndrome due to coronavirus 2 (SARS-COV-2) based on reverse transcription-polymerase chain reaction (RT-PCR) (11). On the other hand, some studies have debated no vertical transmission of COVID-19. A narrative review study pointed out the lack of adequate data on COVID-19 during pregnancy and recommended extensive follow up of mothers and fetuses (10). Favre et al. (2020) insisted on the current lack of data on the consequences of COVID-19 and recommended extended follow-up for infected pregnant women and their fetuses (8).
However, confirmed evidence on the COVID-19 vertical transmission are crucial and essential in decision-making for the management of pregnant women with COVID-19 infection. Therefore, this systematic review and meta-analysis aimed to assess the possibility of vertical transmission of COVID-19 from infected pregnant women to fetuses.
2. Objectives
In this systematic review, we followed the Preferred Reporting Items for Systematic Review and Meta-Analysis guideline (PRISMA) (12).
3. Methods
3.1. Search Strategy
The international bibliographic databases including PubMed and SCOPUS as well as Google Scholar were searched to identify relevant studies using the following keywords, which were selected based on medical subject heading (MeSH) terms along with free text searching in combination with Boolean operations (AND and OR) (Table 1).
| Keyword | Search Terms |
|---|---|
| Coronavirus 2019 | COVID 19; COVID 19 infection; COVID 19 outbreak; COVID 19 pneumonia; severe acute respiratory syndrome coronavirus 2; severe acute respiratory syndrome coronavirus infection; severe acute respiratory syndrome coronavirus SARS Cov; severe acute respiratory syndrome coronaviruses; severe acute respiratory syndrome SARS coronavirus |
| Vertical transmission | vertical transmission; vertical transmission rate; vertical transmission rates; vertical transmission risk; vertical transmission risks; vertical transmission route; vertical transmission studies; vertical transmission study; vertical transmissions; vertical transplacental transmission |
The primary search was conducted on PubMed and the findings were used to improve the search. The final keywords and search terms were used for searching other databases. We also searched the WHO website (www.who.int) using the selected keywords. Furthermore, the reference list of the identified studies was checked manually for similar studies.
3.2. Inclusion and Exclusion Criteria
All observational studies, including cross-sectional, cohort, case-control, case reports, and case series, published in peer-reviewed journals until the end of June 2020 were reviewed. Editorials, commentaries, and letter to editors were excluded.
The inclusion criteria were as follows: 1- human studies, 2- women who gave birth to a live child in the past 3 months, 3- women and neonates who were tested positive for COVID-19, 4- Studies that included neonates with mothers who were tested positive for COVID-19.
Articles in any language were included. Studies with low quality were excluded from the review due to their negative effects.
The exposure was considered as infection with SARS-COV-2 virus documented by positive COVID-19 RT-PCR (13). The sensitivity for RT-PCR in detecting COVID-19 infection was previously reported to range between 95% and 100% (1, 13). All subjects with at least two positive RT-PCR results for COVID-19 were considered as COVID-19 infected. This diagnosis was implied for both mothers and newborn infants.
The RT-PCR is not performed routinely for all pregnant mothers, and only those who presented with suspicious clinical signs and symptoms for COVID-19 infection or those with radiological findings for COVID-19 infection are tested using RT PCR.
3.3. Study Selection
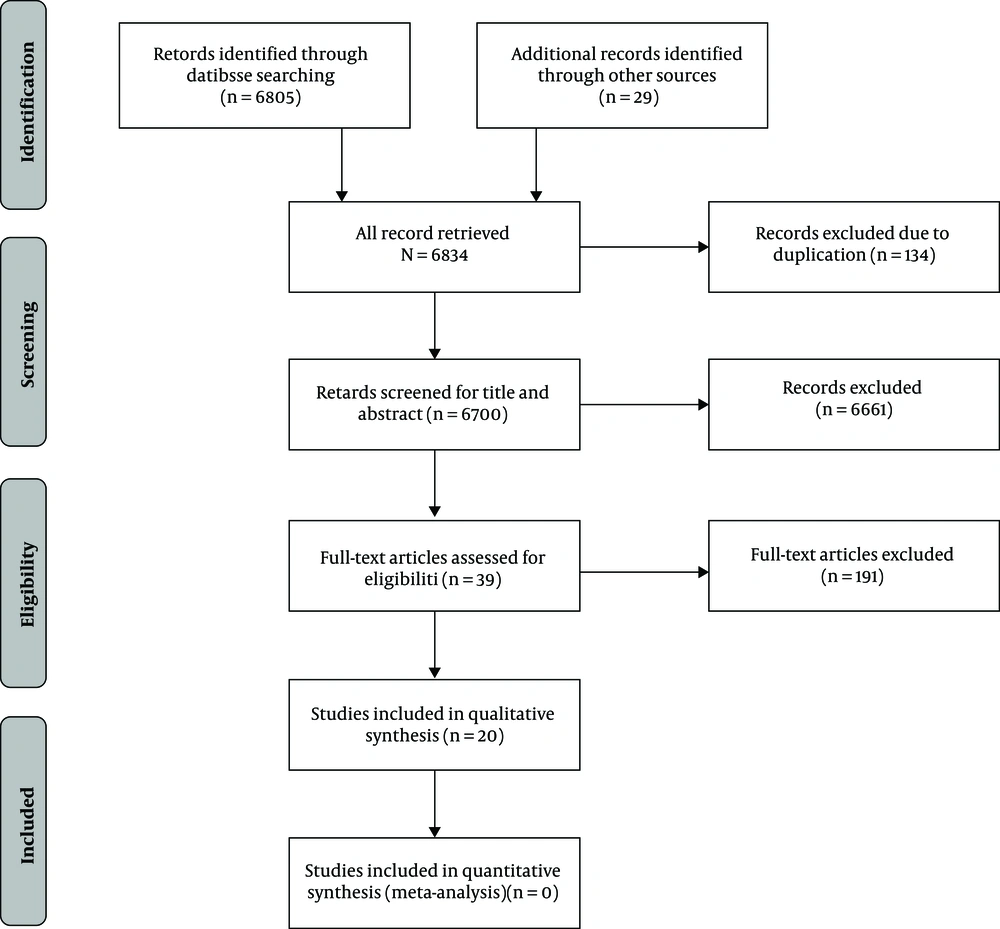
A total of 683400 articles were retrieved, including 71 articles from PubMed, 34 from Scopus, and 6700 from Google Scholar, as well as 29 articles, which were identified through reviewing references of retrieved studies. Then, 134 articles were excluded due to duplication (that is, 6700 articles remained). After assessing titles and abstracts, 6661 articles were excluded based on the inclusion criteria, and 39 articles remained for full-text assessment. Nineteen publications were excluded after applying inclusion criteria in the full-text evaluation. Finally, 20 studies were potentially eligible. The flowchart of the study selection process is shown in Figure 1.
The Zotero reference manager (https://www.zotero.org/) was used to merge the identified studies and to remove duplicate publications as well as screening the titles and abstracts. The identified studies were assessed by two authors independently based on titles and abstracts. Eligible studies were selected for the review. In case of a disagreement, a consensus was reached through discussion or, if necessary, the third reviewer was consulted.
3.4. Data Extraction
All authors contributed to data extraction from the included studies. Extracted data were summarized in an author-developed checklist as a guide, which included the following items:
1- General characteristics of the study (author names, title, publication date, and review date)
2- Type of the study
3- Sample size
4- Study subject characteristics (demographic characteristics, predisposing conditions, gestational age)
5- Measured outcomes and analyses (the diagnostic test used, number of positive samples)
6- Findings.
3.5. Quality Assessment
As findings of systematic reviews may be affected by possible biases, the quality of eligible studies was assessed, regardless of the aim of the study, using the Newcastle-Ottawa Quality Assessment Scale for cross-sectional studies (14, 15), Newcastle-Ottawa Quality Assessment Scale for cohort studies (16), and JBI Critical Appraisal Checklist for case report and case series studies (17, 18). The studies were stratified in three levels based on the quality assessment score as follows: 1- 'High-quality studies' determined as studies that obtained at least 75% of the maximum attainable score, 2- 'Moderate quality studies' determined as studies that obtained 50% to 75% of the maximum attainable score, 3- 'Low-quality studies' determined as studies that obtained less than 50% of the attainable score. Therefore, the 50% cut-off was used to exclude low-quality articles. Methodology quality assessment of included studies is shown in Table 2.
| Authors | Study Design | Sample Size | Gestational Age (Weeks) | Delivery Method | Tests to Assess Mother-To-Child Transmission | Tests Results | Newborn Prognosis | Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| 1. Chen h. et al. (10) | Retrospective | 9 | 36 weeks and above | C-section | Amniotic fluid, cord blood, neonatal throat swab, breastmilk samples RT-PCR | 100% negative | Unavailable information | Moderate |
| 2. Zhang l et al. (19) | Retrospective | 16 | 35 weeks and above | C-section | Neonatal oropharyngeal swab RT-PCR | 100% negative | Unavailable information | High |
| 5. Zhu h et al. (11) | Retrospective | 10 | 40% full-term infants 60% premature infants | 98% Cesarean section and 2% vaginal | Neonatal oropharyngeal swab | 100% negative | 50% discharged, 40% hospitalized, and 10% death | High |
| 6. Zeng et al. (20) | Cohort study | 33 | 12% premature infants 88% full-term | 78% Cesarean section (N = 26) 22% vaginal (N = 7) | Neonate oropharyngeal and anal swab RT-PCR at 2, 4 and 6 days after birth | 91% negative (N = 30) 9% positive (N = 3) | Stable vital signs or discharged | High |
| 9. Chen S et al. (21) | Retrospective | 5 | 38-41 weeks | 60% vaginal (N = 3) 40% C-section (N = 2) | Neonate throat swab RT-PCR | 100% Negative | Discharged | Moderate |
| 11. Zeng H et al. (22) | Retrospective | 6 | 40 weeks | Cesarean section | Neonatal blood and throat swab, Quantitative RT-PCR on neonatal serum and throat swabs, IgG and IgM antibodies | RT-PCR was negative in all infants. Virus-specific, antibodies were detected in all of them. Two infants had elevated IgG and IgM concentrations | Discharged | High |
| 14. Yang et al. (9) | Prospective | 7 | 36- 40 weeks | Cesarean section | Umbilical cord blood, amniotic fluid and pharyngeal swabs | All RT-PCR tests were negative | Discharged | Moderate |
| 16. Yu N. et al. (23) | Retrospective | 7 | 37 – 41 weeks | Cesarean section | Throat swab specimens, placenta and cord blood | RT-PCR test was positive in one of neonates | Discharged | High |
| 20. Wei Liu et al. (24) | Prospectively | 19 | 38.6 ± 1.5 weeks | 18 Cesarean section and one vaginal delivery | Throat swab, gastric fluid, urine and feces | All of neonatal were negative except one of them was positive in SARS-CoV-2 RT-PCR in throat swab and repeated test was negative. Amniotic fluid and umbilical cord blood test were negative. | High |
The quality of the articles was checked by the two authors. In case of disagreements, discrepancies were resolved through discussion. If the issue remained unresolved, the first author was consulted to resolve any conflict.
4. Results
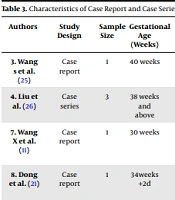
A total of 20 articles, including six retrospective studies (10, 11, 19, 22, 23), 2 prospective studies (9, 24), one cohort (20, 24), nine case reports, and two case series were included in the review (9, 11, 21, 25-31). The overall characteristics of eligible articles are presented in Tables 2 and 3.
| Authors | Study Design | Sample Size | Gestational Age (Weeks) | Delivery Method | Tests to Assess Mother-To-Child Transmission | Tests Results | Newborn Prognosis | Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| 3. Wang s et al. (25) | Case report | 1 | 40 weeks | C-section | Amniotic fluid, cord blood, neonatal throat swab, breastmilk samples RT-PCR | Throat swab positive (36h of age). All other samples negative | Discharged | High |
| 4. Liu et al. (26) | Case series | 3 | 38 weeks and above | 66% Cesarean section and 33% vaginal | Neonatal oropharyngeal swab, blood, cord blood, urine and feces RT-PCR | 100% negative | Discharged | High |
| 7. Wang X et al. (11) | Case report | 1 | 30 weeks | Cesarean section | Neonatal oropharyngeal swab and stools, amniotic fluid, cord blood, placenta RT-PCR | Negative | Discharged | Moderate |
| 8. Dong et al. (21) | Case report | 1 | 34weeks +2d | Cesarean section | Nasopharyngeal swab, maternal vaginal secretions and breastmilk RT-PCR; specific maternal and neonatal IgG and IgM | Positive IgG and IgM count Negative nasopharyngeal swab of neonate and vaginal secretions | Hospitalized | High |
| 10. Fan et al. (27) | Case report | 2 | 36–37 weeks | Caesarean section | Nasopharyngeal swab, maternal serum, vaginal swab, and breast milk, placenta tissues, umbilical cord blood, amniotic fluid | 100% negative | Discharged | Moderate |
| 12. Khan et al. (28) | Case series | 17 | 35-41 weeks | Cesarean section | RT-PCR or CT scan imaging | Two neonates were suspected and five neonates were reported with neonatal pneumonia. | Neonatal pneumonia occurred in five of the 17 neonates | High |
| 13. Lu et al. (29) | Case report | 1 | 38 weeks | Cesarean section | Nasopharyngeal swabs, oropharyngeal swabs, and blood sample | All RT-PCR tests were negative | Discharged | High |
| 15. Liu Y. et al. (32) | Case report | 13 | 25 weeks and above | Cesarean section | Oropharyngeal swabs | All RT-PCR tests were negative | No severe neonatal asphyxia was observed, No vertical transmission | High |
| 17. Zambrano et al. (30) | Case report | 1 | 32 weeks | Vaginal delivery | Nasopharyngeal and blood sample | RT-PCR tests were negative | Hospitalized | Moderate |
| 18. Li Yet al (9) | Case report | 1 | 35 | Cesarean section | Oropharyngeal swab specimen | RT-PCR was negative | Unavailable information | Moderate |
| 19. Xiong et al. (31) | Case report | 1 | 33 | Vaginal delivery | Amniotic fluid, neonatal throat, swab | RT-PCR were negative | Discharge | High |
The presence of SARS-CoV-2 was assessed in oro/nasopharyngeal swab (9-11, 19-32), cord blood (9-11, 23, 25-27), amniotic fluid (9-11, 25, 27, 31), neonate blood (21, 22, 26, 29, 30), breast milk (10, 21, 25, 27), urine and feces (11, 24, 26), placenta (11, 23, 27), maternal blood (21, 27), maternal vaginal secretions (21, 27), anal swab (20), and gastric fluid (24). The overall cases in the reviewed articles summed up to 154. Vertical transmission was reported in 9 cases (5.8%). Detection of vertical transmission was based on RT-PCR (n = 6, 3.9%) and serum antibodies (n = 3, 1.9%).
Only one cohort study (n = 33) was included in this review, which reported vertical transmission in 3 (9%) of the cases based on RT-PCR (20). Among 8 retrospective studies, with overall 79 cases, the vertical transmission was reported in 3 studies (overall 4 cases) based on the presence of serum antibody (2 cases) (22) or positive throat swab by RT-PCR (21, 22). Of 11 case reports/series, with overall 42 cases, the vertical transmission was reported in one article (one case) based on positive throat swab using RT-PCR (25), and in one article (one case) based on serum antibody (21), while in one case series study the presence of SAR-CoV-2 was not documented in two suspicious cases (28).
The primary objective of the present systematic review was to assess the possibility of vertical transmission of COVID-19 from infected pregnant women to fetuses during either pregnancy or labor. We reviewed 20 eligible articles, and the likelihood of vertical transmission was found to be very low based on the RT-PCR. The type of delivery was a Caesarean section (C-section) in the majority of cases. The reasons for performing C-section included poor clinical condition of the mother, obstetrics complications, and in some studies, fetal distress. Therefore, the high incidence of C-sections might be attributed to the mentioned complications. Elective C-section was not performed in any cases in this review. Fetal age in the reviewed studies ranged from 25 to 41 weeks. In a retrospective study by Zhu et al., 10% of the newborns from COVID-19 infected mothers died. On the other hand, 60% of the neonates were preterm, and 100% of the neonates had negative COVID-19 test results, the mortality rate could be attributed to prematurity.
Various diagnostic tools, including serum IgM and IgG, assessment of virus presence in neonatal nasopharyngeal secretions using RT-PCR, cord blood samples, nasopharyngeal smears, lung scan, assessment of virus presence in breast milk and amniotic fluid have been used for detecting COVID-19 infection in the newborns from infected mothers.
The rate of negative results in RT-PCR tests ranged between 82% and 100%. Although Yu et al. reported positive RT-PCR in one out of 7 neonates, all neonates did not require admission and were discharged from the hospital.
Based on the findings of a study by Yang et al. (2019) on 1014 COVID-19 infected Chinese subjects, the sensitivity of RT-PCR was lower than lung CT-scan (9). Fang et al. (2020) reported that the sensitivity of lung CT-scan and RT-PCR for the diagnosis of COVID-19 were 98% and 78%, respectively (33).
In this systematic review, lung CT-scan was only performed in one study (i.e., Khan et al.), and the majority of the studies have used primary RT-PCR and immunoglobulin assessment as diagnostic markers (28).
Furthermore, a few neonates, 6 (out of 154) live births, in the included studies, had positive RT-PCR results in nasopharyngeal smears (20, 22-25). Also, based on the findings, all neonates with positive RT-PCR results were discharged, but no follow up data was available. Three (1.9%) neonates were reported to have positive IgG or IgM without positive RT-PCR results (21, 22).
In this systematic review, the most important gap in the included studies was the lack of laboratory and clinical follow-up data. Another gap was not following up infected mothers. It should be noted that the lack of follow up was due to the nature of the studies (i.e. retrospective and case report/series).
One of the limitations of this study was the heterogeneity in the methodology of eligible articles that prevented us from performing a meta-analysis. Furthermore, due to the moderate quality of some studies, there was a possibility of bias in the studies, which might affect the conclusions of this review. Further studies are needed to make a conclusion regarding the possibility of vertical transmission of COVID-19 with a higher confidence level, particularly prospective studies. This study can be considered as one of the few studies that have reviewed published articles in the first 5 months from the initiation and spread of the COVID-19 pandemic. In the present study, two researchers extracted data simultaneously in order to minimize the potential reviewer bias. Furthermore, the study protocol was registered in the Prospero website, which validates the strategy of this research.
5. Conclusions
This systematic review demonstrated a low risk of vertical transmission of COVID-19 (less than 10%) from infected pregnant women to fetuses so that only the RT-PCR results of 7 (out of 145) neonates born to infected mothers was positive. It worth noting that the majority of these neonates were discharged from the hospital. However, some of them were hospitalized because of prematurity. But there is still a need for further studies with larger sample sizes. Regarding the short period from the onset of the COVID-19 pandemic and the scarcity of publications regarding the vertical transmission of the disease, we didn't have any option except to include case reports and case series in this review. We suggest performing further epidemiologic studies.