1. Introduction
Since the novel coronavirus disease 2019 (COVID-19) has emerged, it has infected 10,922,324 people around the world, with 523,011 deaths globally (1). This disease that is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has different manifestations, mainly including respiratory and systemic illness, which can result in acute respiratory distress syndrome (ARDS), intravascular coagulopathy, and multi-organ failure in critical cases (2). It has been reported that children who are infected with SARS-CoV-2 are often asymptomatic, and among the symptomatic cases, the major manifestations are fever (46%) and cough (42%), while other clinical manifestations such as diarrhea, vomiting, fatigue, and nasal congestion are observed in approximately 10% of the infected children (3). Some common hematological manifestations of COVID-19 include lymphopenia and thrombocytopenia with normal white blood cell (WBC) count (4). Studies have shown that coagulation dysfunction is common in deaths caused by SARS-CoV-2 pneumonia (5). As thrombocytopenia can be a common laboratory finding in viral infections such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), varicella-zoster virus, influenza, and human immunodeficiency virus (HIV) (6, 7), it can also occur with SARS-CoV-2 (8). Since there are a few reports of immune thrombocytopenic purpura (ITP) associated with COVID-19 in children, in this study, we describe a case of pediatric SARS-CoV-2 positive patient with ITP.
2. Case Presentation
A previously healthy 7-year-old boy presented on May 25, 2020 with a 4-day mild gingival bleeding and ecchymosis on both lower extremities with no history of trauma. He was the second child of the family and born by normal vaginal delivery, full-term, with no remarkable antenatal and perinatal history and closely-related parents. There was no history of fever, cough, nausea and/or vomiting, diarrhea, headache, vertigo, and urine discoloration. He was admitted with suspicion of ITP, and a low platelet count was found in his lab data. Bone marrow aspiration and peripheral blood smear (PBS) were both normal and suggestive of ITP. His father had a history of fever, cough, and bone pain 2 weeks prior to the case’s admission with no evidence of hemorrhage, petechiae, or purpura but leukopenia, lymphopenia, and low platelet upon laboratory results. In addition, his sister had a history of petechiae and ecchymosis with mild gingival bleeding 4 days before the appearance of symptoms in our case. Her chest CT scan was normal, and she showed negative results in two separate reverse transcriptase polymerase chain reaction (RT-PCR) tests for COVID-19. His father and sister were both diagnosed as possible ITP cases based on their bone marrow aspiration. BPS had not been performed for the father and sister.
On admission (May 25, 2020), the vital signs were normal, and on physical examination, the patient appeared well with petechial lesions on the hard palate on oral observations. Skin examination showed petechiae scattered on his knees and ecchymosis on both his legs and ankles. Cardiac, pulmonary, abdominal, neurological, lymph node and other physical examinations were normal. In an outpatient visit by a general practitioner on May 23, 2020, laboratory work-up had been requested. The results showed significant thrombocytopenia with a platelet count of 8,000/µL associated with WBC count of 4400/µL, red blood cell (RBC) count of 4.77 × 106/µL, hemoglobin (Hb) of 10.1 g/dL, hematocrit (HCT) of 32.1%, mean corpuscular volume (MCV) of 67.3 fL, and normal partial thromboplastin time (PTT), prothrombin time (PT), and international normalized ratio (INR). Abdominopelvic sonography was normal with no organomegaly. Also, the reticulocyte count was 1.2%, and direct and indirect Coombs tests were both negative. PBS showed hypochromia and poikilocytosis in addition to 5% atypical lymphocytes with other features described as normal.
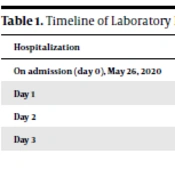
On May 26, 2020, based on the patient’s severe thrombocytopenia and absence of fever, organomegaly, or lymphadenopathy, in the setting of suspected ITP, he was admitted to the hematology service of Bandar Abbas Pediatric Hospital affiliated to Hormozgan University of Medical Sciences, Iran. Intravenous immunoglobulin (IVIG) with a total dose of 2 g/kg was administered for 2 days. The complete blood count (CBC) was repeated after 2 days on May 28, 2020 and showed a platelet count of 20000/µL. The timeline of laboratory results and treatment is demonstrated in Table 1. On May 28, 2020, his father’s nasopharyngeal swab turned out positive for SARS-CoV-2 on RT-PCR testing. The delay in RT-PCR testing of the father and his evaluation regarding COVID-19 was due to the low socioeconomic status of the family and lack of health insurance. Due to the similarity of hematological findings of low platelet count in the patient's father, the patient was transferred to the COVID-19 ward, and COVID-19 diagnostic testing was performed. Chest computed tomography (CT) was also performed, which was unremarkable.
| Hospitalization | WBC (/µL) | Hb (g/dL) | Plt (/µL) | Lymphocyte (%) | Neutrophil (%) | PT (s) | PTT (s) | INR | Started Treatment |
|---|---|---|---|---|---|---|---|---|---|
| On admission (day 0), May 26, 2020 | 4400 | 10.1 | 8000 | - | - | 12.3 | 33.5 | 1 | - |
| Day 1 | 3300 | 9.2 | 36000 | 64.5 | 25.2 | - | - | - | - |
| Day 2 | 3100 | 8.9 | 20000 | 69.7 | 15.2 | - | - | - | IVIG * 2 days |
| Day 3 | 2600 | 9.4 | 41000 | 69 | 20.1 | - | - | - | - |
Upon visiting the patient at the COVID-19 ward on May 29, 2020, PT, PTT, INR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), thyroid function tests (T4 and thyroid stimulating hormone [TSH]), anti-EBV IgM, anti-CMV IgM and IgG, anti-HIV antibody (Ab), anti-HCV Ab, and H. pylori stool antigen were requested. The results showed PT of 11.7 s, PTT of 27 s, INR of 1, AST of 59 U/L, ALT of 117 U/L, ALP of 644 U/L, creatinine (Cr) of 0.55 mg/dL, urea of 32 mg/dL, blood urea nitrogen (BUN) of 15 mg/dL, sodium of 138 mEq/L, potassium of 4 mEq/L, lactate dehydrogenase (LDH) of 468 U/L, troponin < 1.5 ng/mL, ferritin of 262 ng/mL, C3 of 107 mg/dL, C4 of 13 mg/dL, negative antinuclear antibody (ANA), negative anti-double stranded DNA (anti-dsDNA), T4 of 6.8 µg/dL, and TSH of 2.66 mIU/L. Anti-CMV IgM and anti-EBV IgM were negative (0.06 and 0.02, respectively), but anti-CMV IgG was positive. The results for anti-HIV Ab and anti-HCV Ab were negative. CBC was also rechecked. The results showed the worsening course of WBC, which decreased to 2600/µL (20.1 % neutrophil and 69% lymphocyte), RBC of 4.22 × 106/µL, Hb of 9.4 g/dL, HCT of 48.2%, and platelet count of 20000/µL. Therefore, due to the decline of the WBC count and Hb level, and because platelet count had not reached the acceptable level, a hematologic consult regarding bone marrow aspiration and biopsy was requested, which turned out normal. Besides, his nasopharyngeal swab came out negative for SARS-CoV-2 on RT-PCR testing on May 29, 2020, that was rechecked based on high suspicion for COVID-19. Also, anti-SARS-CoV-2 IgM and IgG were both in the negative range, and the results of flow cytometry were unremarkable.
The patient was discharged on the 3rd day of admission to the COVID-19 ward (on June 1, 2020) with 5 mg oral prednisolone QID and 100 mg vitamin E QID. The patient was asked to recheck CBC, differentials, liver function tests, LDH, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and uric acid after one week as a follow-up plan.
Four days after being discharged, on June 5, 2020, the second nasopharyngeal swab for SARS-CoV-2 on RT-PCR testing came out positive; Thus, the laboratory tests were sent on the same day, and based on the platelet count of 3000/µL, the patient was readmitted. On admission, he looked well and asymptomatic with stable vital signs. Physical examination was normal except for ecchymosis on the left leg and flank and some petechial lesions on the left knee. Methylprednisolone pulse therapy with a total dose of 30 mg/kg/day for three days and oral hydroxychloroquine with a total dose of 4 mg/kg/day twice a day for 5 days were administered. Given the unresponsiveness of the patient to the primary treatment (IVIG and prednisolone), repetitive platelet reduction, and the possible activity of SARS-CoV-2, we decided to administer antivirals for the patients. After three days of treatment with methylprednisolone, CBC was rechecked, and the platelet count had increased to 8000/µL; thus, he was discharged on June 8, 2020, with 25 mg oral prednisolone twice a day. Other test results during the patient’s second admission are presented in Table 2. Also, AST of 32 U/L, ALT of 64 U/L, ALP of 482 U/L, negative Coombs test, negative anti-HAV IgM, and normal G6PD level were reported. It appears that the abnormal LFT on first admission may have been due to the immune response to COVID-19 infection. Although LFTs were still abnormal on the second admission, the liver enzymes had declined compared to the first admission. We had suspected the potential causes of elevation of liver enzymes such as CMV and EBV; however, the patient had tested negative for both. The patient was followed up by rechecking CBC and liver function tests on June 22, 2020, in an outpatient visit. The platelet count was elevated to 20000/µL, and his general condition was good with no evidence of hemorrhage or skin lesions. The dose of prednisolone was tapered to 5 mg twice a day. Another CBC and SARS-CoV-2 on RT-PCR testing will be requested in a follow-up visit.
| Hospitalization | WBC (/µL) | Hb (g/dL) | Plt (/µL) | Lymphocyte (%) | Neutrophil (%) | ESR (mm/h) | CRP (mg/L) | LDH (U/L) | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| On admission (day 0) June 5, 2020 | 4100 | 8.4 | 3000 | 60.6 | 28.2 | 49 | 5.7 | 272 | 32 | 64 | 368 |
| Day 2 | Started treatments: Methylprednisolone pulse therapy*3days; Hydroxychloroquine*5days | ||||||||||
| Day 4 | 4200 | 7.8 | 8000 | 46.2 | 45.8 | - | - | - | - | - | - |
3. Discussion
In this case report, we described a pediatric case of COVID-19 who presented with hemorrhagic symptoms (petechiae, epistaxis) very likely caused by immune thrombocytopenia. The current COVID-19 pandemic, caused by the novel SARS-CoV-2 in December 2019, is mainly characterized by respiratory symptoms ranging from a mild involvement of the upper respiratory tract accompanied by fever (82%) and cough (81%) to ARDS and sepsis. However, there are no laboratory markers for the evaluation of the disease severity. In COVID-19 patients, the number of platelets is currently a concern (9). Some studies suggested that a low platelet count is associated with a higher mortality rate (10). Although in most patients, the platelet count does not decrease as low as the level at which bleeding occurs, there are a few reports on how this virus can interfere with the hematopoietic system, which are summarized as: (1) direct viral involvement of bone marrow cells; (2) immune-mediated platelet destruction; and (3) aggregation of platelets in the lungs leading to the consumption of platelets (4). Furthermore, it has been reported in a recent study that 69% of patients with SARS-CoV-2-induced ITP had bleeding tendency; cutaneous manifestations in the form of petechiae, purpura, and ecchymosis were observed in 51% of the patients, while among other forms of bleeding, epistaxis was the most common. In addition, intracranial hemorrhage (ICH) was found in four patients (7.8%), as well as small intraventricular hemorrhage and subarachnoid hemorrhage in 4% (11). Hemorrhage in terms of ICH can lead to mortality in SARS-CoV-2-induced ITP as one of the four patients with ICH had died (11).
There are a few reports describing immune thrombocytopenia in pediatric COVID-19 patients. In a recent systematic review, the median age of patients with SARS-CoV-2-induced ITP was 62 years, and only 7% were in the pediatric age group (11). Hereby, we reported the presentation, work-up, and treatment options in a SARS-CoV-2-positive child with SARS-CoV-2-induced ITP whose father had possible SARS-CoV-2-induced ITP. His sister also had mild hemorrhagic symptoms and confirmed ITP based on her peripheral blood smear and bone marrow biopsy; however, she tested negative for SARS-CoV-2 RNA and antibodies. Furthermore, there was no history of underlying familial thrombocytopenia that could justify the findings in our case and his close relatives. Although our case presented with no COVID-19 symptoms, this may not be the case in other SARS-CoV-2-induced ITP patients. The median time between the onset of COVID-19 symptoms and the diagnosis of SARS-CoV-2-induced ITP has been reported to be 13 days, with the majority of cases occurring during the second and third weeks of the onset of COVID-19 symptoms (11). Nonetheless, reports of ITP in asymptomatic COVID-19 patients underscores the need for COVID-19 testing in newly diagnosed ITP patients amid this pandemic.
It appears that our patient did not respond to IVIG. Instead, he had a good response to corticosteroids, contradictory to previous studies in which IVIG was suggested as the first-line treatment (12-16). However, in a recent study, it was suggested that steroids may be a better option for the initial treatment of COVID-19 patients presenting with new or relapsing ITP and that IVIG may be used as the second-line treatment if there is failure to respond to steroids (17). In a previous study conducted in the United States, Tsao et al. described the first case of a pediatric COVID-19 patient with ITP. A 10-year-old previously healthy female presented with one day of rash and a history of mild illness who had negative family history for hematologic or autoimmune disorders responded rapidly to IVIG with blood counts normalizing within 2 weeks, which is in contrast to our study (16). In another study, authors reported a 12-year-old female without any significant past medical history who presented cough, fever, and vomiting. Laboratory evaluations showed severe thrombocytopenia, and test results for SARS-CoV-2 returned positive. This patient was also treated as ITP with IVIG and corticosteroids and had a good recovery regarding her platelet count (14). In comparison to our study, this case progressed to a more severe disease, and her ITP was treated with IVIG. The authors of a study published in May 2020 reported three adult patients with severe thrombocytopenia during COVID-19 infection accompanied by mucosal bleeding or cutaneous purpura. One patient recovered spontaneously; however, two others received IVIG and eltrombopag (13), which is in contrast to our results. In another case series of three adult COVID-19 patients, they presented with thrombocytopenia, and a quick response to IVIG was reported (12).
To the best of our knowledge, this is the only case of ITP within the course of COVID-19, which was resistant to IVIG while responded to corticosteroids. We speculate that the thrombocytopenia in the course of the disease is secondary to the viremia, as a result, by using antiviral agents, platelet count elevation is observed. However, we suggest further investigation in this area. Moreover, it appears that some COVID-19 phenotypes are more prone to SARS-CoV-2-induced ITP as demonstrated by Bhattacharjee and Banerjee (11). They showed that 75% of patients with SARS-CoV-2-induced ITP had moderate-to-severe COVID-19. Mild COVID-19 patients were reported in 18%, and 7% did not give any history of fever or other typical flu-like symptoms; therefore, patients with moderate-to-sever COVID-19 appear to be more susceptible to SARS-CoV-2-induced ITP compared to others (11).
In conclusion, the case reported in the current study highlights the need to be vigilant for atypical presentations or complications of COVID-19, such as SARS-CoV-2-induced ITP. It is necessary to pay attention to platelet count in addition to typical clinical features and radiographic findings. On the other hand, viral testing in thrombocytopenic patients should be considered for the timely diagnosis of COVID-19 and taking necessary measures in addition to patient isolation and treatment of COVID-19 in order to prevent the spread of disease and infection of healthcare workers during this pandemic.