1. Background
Streptococcus pyogenes Group A Streptococcus (GAS) is one of the foremost clinically important Gram-positive pathogens causing a wide spectrum of human infections. It causes diseases ranging from relatively benign infections, such as sore throats, to severe invasive diseases, like streptococcal toxic shock syndrome and acute rheumatic fever (ARF) (1, 2). The rate of mortality of invasive GAS infections, especially those with ARF, is high in developing countries, and the majority of them develop rheumatic heart disease (RHD) (1, 3, 4). In Morocco, two-thirds of children with ARF have a history of sore throat, of whom 53.1% develop RHD and 2.7% die (4).
GAS is responsible for 15 - 30% of acute pharyngitis cases in children (5, 6). This elevated prevalence may be due to the changes in specific virulence factors of GAS. Several outbreaks of a rare serotype of GAS pharyngitis and invasive GAS infections have been reported (7-9). Cell-surface M protein is the main determinant of the virulence of GAS (2). The M protein is encoded by the emm gene and has a hypervariable region of 40 to 50 amino-terminal acid residues (10-12). The sequencing of this region of the M protein constitutes the GAS typing system and it is named emm typing. It is the currently accepted reference standard methodology used for characterizing GAS isolates and identifying the different emm types (13).
Nowadays, more than 170 emm types and 750 emm subtypes of GAS have been documented (14). The distribution of emm types is different depending on the countries and regions (2, 15). Therefore, typing of streptococcus pyogenes isolates in each region is essential as part of the epidemiological surveillance for the disease (6, 16).
Regarding the treatment of GAS infections, it is based on beta-lactams, especially the penicillin G that presents the treatment of choice for doctors because of its effectiveness, cost, and narrow spectrum of activity. Erythromycin and first-generation cephalosporin are recommended for patients with penicillin allergies (17, 18). However, inadequate treatment of pharyngitis and specially GAS pharyngitis can cause severe diseases. This is why it is important to know the pathogens responsible for pharyngitis and their virulence in order to reduce the risk of ARF and other post-streptococcal infections.
2. Objectives
To our knowledge, this is the first study in Morocco that gives an idea about the different types of M protein in GAS isolated from children with pharyngitis. The main aim of this exploration was to determine the prevalence of GAS pharyngitis in children with throat infections and know the different types of their M proteins.
3. Methods
3.1. Patients and Samples
This cross-sectional descriptive study was done for 14 months (from February 2017 to March 2018). The samples were taken from the throats of children with pharyngitis, consulting a primary health care center in Fez, Morocco.
The patients who participated in this study were diagnosed by their doctors with throat infections. We included all children who were presented with an erythematous or erythematous pultaceous sore throat, fever, and headache. Patients with other respiratory tract symptoms and those with prior antibiotic therapy (in the last seven days) were excluded. A throat swab was considered the technique of sampling in this study. The swab collects a sample of the secretions produced in the back of the throat. To decrease technical bias, the back of the throat should be clearly seen. If the tongue and/or the cheeks are touched, the throat swab is remade. The tube should be sealed tightly.
We recorded the demographic and clinical data of patients, including the age of children, gender, and disease onset. The symptoms were also registered. The data were collected after an unwritten agreement and explanation about the purpose of the study. Microbial analysis was performed at the laboratory of microbiology and molecular biology, Fez Medicine School. For quality control, we used a collection of GAS strains already isolated in our laboratory.
3.2. Phenotypic GAS Characterization
After the collection of the throat swabs from the children included in the study, it was immediately placed in brain heart infusion broth (BHI) (Oxoid, UK). Later, the samples were incubated at 37°C for 24 hours in the laboratory oven.
The next day, a subculture was carried out on blood agar plates with 5% CO2 at 37°C overnight (Biomérieux, France). To identify the colonies isolated, different characteristics of morphology or growth were considered. The beta hemolysis on blood agar medium, catalase test, Gram staining, and latex agglutination test were the used (Oxoid, UK). The bacitracin susceptibility was determined by the absence of an inhibition zone around the disk of bacitracin (0.04 IU). The sulfamethoxazole–trimethoprim (SXT) resistance was determined by the presence of an inhibition zone around the disk of SXT.
3.3. Antibiogram
To determine the sensitivity/resistance of GAS colonies against different antibiotics, we used the standard disk-diffusion method on Mueller Hinton agar with 5% sheep blood (Biomérieux, France). The colonies were incubated for 24 hours at 37°C with 5% CO2 Jar according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2017). The commercial antibiotic discs (Oxoid, France) were as follows: penicillin G (1 μg), Vancomycin (5 μg), clindamycin, erythromycin (15 μg), gentamicin (10 μg), tetracycline, and rifampicin.
3.4. Detection of the emm Gene
Total DNA was extracted from the GAS isolated and the broth of the throat respectively using a commercial extraction and purification kit (Prep Man) (Applied Biosystems, USA). In order to amplify the emm gene, primers 1 and 2 (http://www.cdc.gov/streplab/protocol-emm-type.html), were used for amplifying the N-terminal region of the emm gene: primer 1: 5-TAT TCG CTT AGA AAA TTA A-3 and primer 2: 5-GCA AGT TCT TCA GCT TGT TT-3.
The PCR reaction mixture included PCR buffer, 1.5 mM MgCl2, 10 mM deoxynucleotide, 70 pM of each primer, 3 U Taq polymerase, and 1 µL of the template DNA (Promega, Madison, USA). The final volume was 25µL. Amplification was performed on a thermocycler Hybaid's PCR Express™. The amplification program for PCR reactions composed of an initial denaturation (94°C, 1 minute), 30 cycles of denaturation (94°C, 15 seconds), annealing (47°C, 30 seconds), extension (72°C, 1 min 15 seconds), and a final extension (72°C, 7 minutes). PCR products were run on 2% agarose gel (FMC BioProducts, Rockland, ME) containing 0.5 mg/mL ethidium bromide (Qiagen) followed by ultraviolet illumination, and were photographed with an Olympus digital camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany). The PCR products were sequenced with the same primers used for PCR amplification to determine the emm sequence type (3130-1 Genetic Analyzer, Applied Biosystems, Foster City, CA). The sequences of emm gene were analyzed using the BLAST program (http://www.ncbi.nlm.nih.gov).
3.5. Data Management and Analysis
Descriptive statistics were used to characterize the respondent’s demographic and clinical characteristics. Frequencies were used for qualitative variables. Means and standard deviations were used for quantitative variables. Statistical analyses were performed using EPIinfo7.
3.6. Ethics Statement
The research protocol was approved by the Ethics Committees of the Hassan II University Hospital Center, Fez, Morocco. Written informed consent was obtained from each child’s parent or legal guardian. The data were analyzed anonymously.
4. Results
The present study was carried out on 177 children with pharyngitis. The age range of the participants was between 5 and 17 years. The mean age was 9 ± 3 years and the sex ratio (male/female) was 1.05. The majority of the cases belonged to a middle-class family (72.3%) and were admitted in autumn and winter (43.5% and 39%, respectively) (Table 1).
| Characteristics | No. of Cases | Percentage of Total |
|---|---|---|
| Gender | ||
| Male | 90 | 50.8 |
| Female | 87 | 49.2 |
| Season | ||
| Autumn | 77 | 43.5 |
| Winter | 69 | 39 |
| Spring | 30 | 16.9 |
| Summer | 1 | 0.6 |
| Education level | ||
| Unschooled | 21 | 11.9 |
| Koranic | 128 | 72.3 |
| Primary | 23 | 13 |
| Secondary | 5 | 2.8 |
| Social level | ||
| Low | 21 | 11.9 |
| Middle | 156 | 88.1 |
| High | 0 |
The majority of children with pharyngitis were presented with a sore throat and pain in swallowing and fever greater than 38°C (91.5% and 90.4%, respectively). The tonsillar exudate was present in 35.6% of children (Table 2). None of the children had developed ARF.
| Symptoms | No. of Cases | Percentage |
|---|---|---|
| Presented Symptoms | ||
| Sore throat and pain in swallowing | ||
| Yes | 162 | 91.5 |
| Total | 177 | 100 |
| Fever | ||
| Yes | 160 | 90.4 |
| Total | 177 | 100 |
| Headache | ||
| Yes | 140 | 79.1 |
| Total | 177 | 100 |
| Nausea and vomiting | ||
| Yes | 60 | 33.9 |
| Total | 177 | 100 |
| Chills and sweats | ||
| Yes | 79 | 44.6 |
| Total | 177 | 100 |
| Presented Sign | ||
| Tonsillar exudates | ||
| Yes | 63 | 35.6 |
| Total | 177 | 100 |
| Earache | ||
| Yes | 24 | 13.6 |
| Total | 177 | 100 |
| Lymphadenopathy | ||
| Yes | 46 | 26 |
| Total | 177 | 100 |
| Rash | ||
| Yes | 2 | 1.1 |
| Total | 177 | 100 |
Among one hundred seventy-seven throat samples obtained from children with pharyngitis, 11 were identified as S. pyogenes by the conventional method (6.2%). The GAS isolated from children belonged to 4 male (36.4%) and 7 female (63.6%) patients. Five out of the eleven children with GAS pharyngitis had tonsillar exudates (45.5%) and the others had pharyngeal erythema. Ten (90.9%) isolates of GAS were sensitive to bacitracin and resistant to SXT. All GAS isolates were sensitive to penicillin and rifampicin, whereas one isolate expressed resistance to erythromycin and clindamycin (9.1%). The results of the antibiogram are presented in Table 3.
| Antibiotics | Sensitive, No. (%) | Resistant No. (%) |
|---|---|---|
| Bacitracin | 10 (90.9) | 1 (9.1) |
| Sulfamethoxazole–trimethoprim | 1 (9.1) | 10 (90.9) |
| Penicillin | 11 (100) | 0 (0) |
| Gentamicin | 10 (90.9) | 1 (9.1) |
| Erythromycin | 10 (90.9) | 1 (9.1) |
| Clindamycin | 10 (90.9) | 1 (9.1) |
| Tetracycline | 9 (81.8) | 2 (18.2) |
| Vancomycin | 9 (81.8) | 2 (18.2) |
| Rifampicin | 11 (100) | 0 (0) |
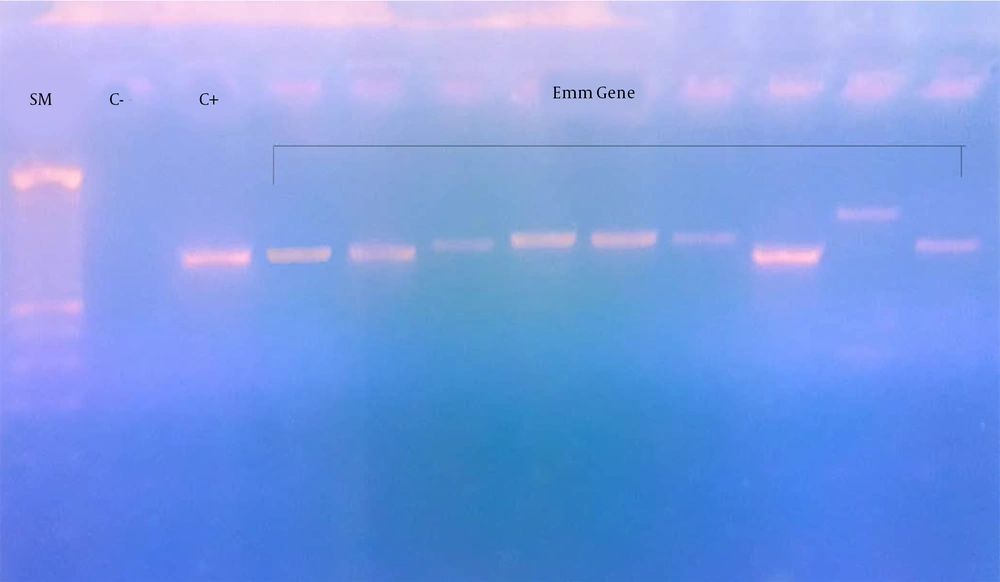
PCR generated 13 different bands of the emm gene: 11 present the GAS isolated previously by the conventional method and 2 obtained from the broth of the throat (Figure 1). In 11 GAS isolates in culture, the BLAST analysis of sequence similarities identified 5 types of emm gene. Two other emm sequence types were identified from the DNA of 2 broths of the throat. The frequency of emm types were as follows: emm type 1, one isolate (7.69%); emm12, two isolates (15.38%); emm22, one isolate (7.69%); emm75, one isolate (7.7%); emm82, one isolate (7.69%); emm89, three isolates (23.08%); and emm90, four isolates (30,77%). Table 4 presents the distribution of emm types according to patients' age and sex. All children with pharyngitis and that presented the type emm 90 were admitted in autumn. The other types of emm were presented in autumn and winter. The distribution of emm types according to the season is presented in Table 5.
| Emm Type | Age (< 15 y), No. (%) | Sex, No. (%) | |
|---|---|---|---|
| Male | Female | ||
| Emm90 | 4 (100) | 3 (75.0) | 1 (25.0) |
| Emm89 | 3 (100) | 1 (33.3) | 2 (66.7) |
| Emm12 | 2 (100) | 0 (0) | 2 (100) |
| Emm1 | 1 (100) | 1 (100) | 0 (0) |
| Emm22 | 1 (100) | 0 (0) | 1 (100) |
| Emm75 | 1 (100) | 1 (100) | 0 (0) |
| Emm82 | 1 (100) | 1 (100) | 0 (0) |
| Emm | Autumn | Winter | Spring |
|---|---|---|---|
| Emm 22 | 0 | 1 | 0 |
| Emm 1 | 1 | 0 | 0 |
| Emm 12 | 1 | 1 | 0 |
| Emm 75 | 1 | 0 | 0 |
| Emm 82 | 1 | 0 | 0 |
| Emm 89 | 1 | 1 | 1 |
| Emm 90 | 4 | 0 | 0 |
| Total | 9 | 3 | 1 |
5. Discussion
Diseases caused by S. pyogenes are a worldwide problem. The determination of GAS prevalence and emm typing is an added value to studies of streptococcal diseases. The M typing system, including emm typing, plays a significant role in the understanding of the GAS epidemiology. As far as we know, this research is the first attempt at S. pyogenes characterization through emm typing in Morocco.
A global estimate of cases of GAS infections was carried out in 2005. It was found that over 616 million incident cases per year of GAS pharyngitis are in developing countries and GAS was considered the 9th leading cause of death due to infection (1). In a study done from March 2006 to February 2007 in four primary health care centers in Rabat and Sale cities in Morocco, the overall prevalence of GAS was 9.3% and it was 9.1% in children (19). In our study, the prevalence of GAS was 6.2%, lower than that estimated by the work of Barbosa and al. (23%) (20). A meta-analysis study found that the prevalence of GAS in 14 studies analyzed was 37%. However, the authors highlighted a high heterogeneity in these studies and the prevalence was ranged from 17% to 58% (21). Another study in Northern India found a prevalence of 2.8 % of children with GAS, which is lower than our results (22).
In addition, emm typing revealed 7 emm profiles and emm90 was the most prevalent type with a frequency of 30.77%, followed by emm89 (23.08%) and emm12 (15.38%). Studies done in Sweden and Brazil reported that just one child with pharyngitis presented the type emm90 (3.1% and 0.92%, respectively), which is lower than our result (23, 24). The studies done by the French National Reference Center for Streptococci and Chen and al. linked this type of emm to invasive GAS infections (25, 26). However, other studies showed that emm90 was involved in a severe epidemic of GAS pharyngitis (7, 16). Few studies found that emm90 is related to GAS pharyngitis because it is a new M type and it is rarely isolated (27).
The distribution of the emm types varies depending on the region. In studies done in Canada, India, and Italy, it was found that emm12 was the most prevalent emm type among children with GAS pharyngitis (28-30). In a study done in Lebanon, Bahnan and al. reported that the most prevalent emm types were emm1, emm22, and emm28 (31). Moreover, in Tunisia, it was shown that emm118, emm42, and emm28 were the most predominant types (32). There were no similarities in the types of emm between different studies and ours. This could be explained by the specific geographic conditions and differences in climate in different regions of the world that may affect the diversity of emm gene (2). We also found that emm 75 had the lowest frequency. This Result is similar to the study in Iran and Tokyo that revealed that emm 75 was the least prevalent isolated emm type (33, 34).
The distribution of the emm types varies depending on the season. In our study, emm 90 and emm75 peaked in autumn. However, in the study done in the US, emm90 (cluster E2) and emm75 (cluster E6) peaked in summer (35, 36). Other studies done in Western Norway and Finland indicated that a peak of GAS incidence occurred during winter and summer. The most presented emm types were emm1 and emm28, while the emm 75 was less presented in this series (37, 38)
Due to the small sample size, the distribution of the emm types according to age was not examined. This is similar to the observation of the study that was done in Japan (34).
As reported by several studies, all our tested isolates were sensitive to penicillin G (18, 28, 31-33). Hence, penicillin remains the drug of choice for treating GAS infections. In case of allergy to penicillin, researchers recommend erythromycin as the best therapeutic alternative for streptococcal infection in the oral cavity. However, over the last few years, some countries reported resistance to erythromycin because of the overuse of the drug. In our study, 9.1% of cases were found resistant to erythromycin, which is lower than the percentage that was found in Brazil, Lebanon, and Spain (15.4%, 10%, and 10.6%, respectively) (18, 31, 39). This percentage of resistance to erythromycin is higher than the observations of the studies that were done in India, Tunisia, Iran, and Turkey (5.6%, 4.8%, 0%, and 3.8%, respectively) (28, 32, 33, 40). Moreover, the studies conducted in Germany and Italy reported an incremental increase of resistance to erythromycin in GAS strains (24% and 35.8%, respectively) (41, 42). GAS still has good sensitivity to penicillin and can be applied in the treatment of bacterial pharyngitis.
Although the ARF cases were not developed in our study, and the complications of tonsillitis are rare, it still presents a risk to acquire the post -streptococcal infections.
5.1. Limitations
This study has some limitations. Firstly, children were diagnosed in just one health center, which indicates that the number of cases included in our study was too small. Secondly, we tested 200 children with sore throats and we tried to exclude 23 those with antibiotic consumption. Finally, some children or their parents refused to make a throat swab sample. Because of these reasons, the number of GAS recovered from this study population was low and we could not find any significant relationship between emm types, and age and antibiotic resistance.
5.2. Conclusions
Despite the fact that the positive GAS number in our work was low, we can conclude that emm90 is the most prevalent type in the region. Also, penicillin and erythromycin are still the treatment choices for GAS pharyngitis cases. The emm typing is useful for molecular studies of GAS and provides new information on the diversity of the emm gene. A higher number of isolates could be considered in the future to validate this genetic diversity. Although this study revealed no complications of GAS pharyngitis, the majority of cases of ARF, mentioned in the last study in Morocco, had a history of acute pharyngitis. Thus, this result indicates the importance of considering GAS pharyngitis in the future and its virulence to prevent children who have many episodes of pharyngitis to develop post-streptococcal complications.