1. Introduction
Coronavirus disease 2019 (COVID-19) has spread throughout the world and was declared a pandemic by the World Health Organization (WHO) (1). Although the proportion of children infected by COVID-19 seems comparatively lower, and the rate of severe symptomatic illness is relatively less than that of adults, the disease in some children could become critical and severe (2, 3).
Severe COVID-19 is characterized by a viral phase and then the cytokine storm, which causes acute respiratory distress syndrome (ARDS), multiorgan failure, and death. Cytokine storm is an uncontrolled systemic inflammatory response resulting from the release of large amounts of pro-inflammatory cytokines and chemokines, which induces an under-recognized condition, namely secondary hemophagocytic lymphohistiocytosis (sHLH). Affected patients present with rapid onset of fever, cytopenia, coagulopathy, elevated transaminase levels, hyperferritinemia, and multiorgan dysfunction (4). Treatment consists mainly of glucocorticoids, intravenous immunoglobulin (IVIG), and chemotherapy (5, 6). Despite these interventions, the mortality rate is as high as 20%. Anticytokine strategies and type I interferons have been proposed to combat the hyperinflammation state, potentially reducing mortality from this condition (7).
In two adults with COVID-19-induced sHLH, the outcomes were poor (8). Recent publications have revealed a manifestation of COVID-19 in children that is similar to Kawasaki disease shock syndrome, but no previous cases in children with COVID-19 have been reported in association with sHLH (3). However, an 18-month-old boy in Isfahan, Iran, has survived COVID-19-induced sHLH. We report the clinical course and therapeutic interventions in this case.
2. Case Presentation
Written informed consent to treatment was obtained from the patient’s parents.
On March 6, 2020, an 18-month-old Iranian child, from Isfahan, without any underlying disease, was admitted to a hospital for high fever, poor feeding, diarrhea, and drowsiness. He had previously been completely healthy; he had become ill seven days after he received routine national vaccinations, which included DPT, MMR, and OPV. The parents reported close contact with COVID-19-confirmed cases in his grandparents three weeks earlier.
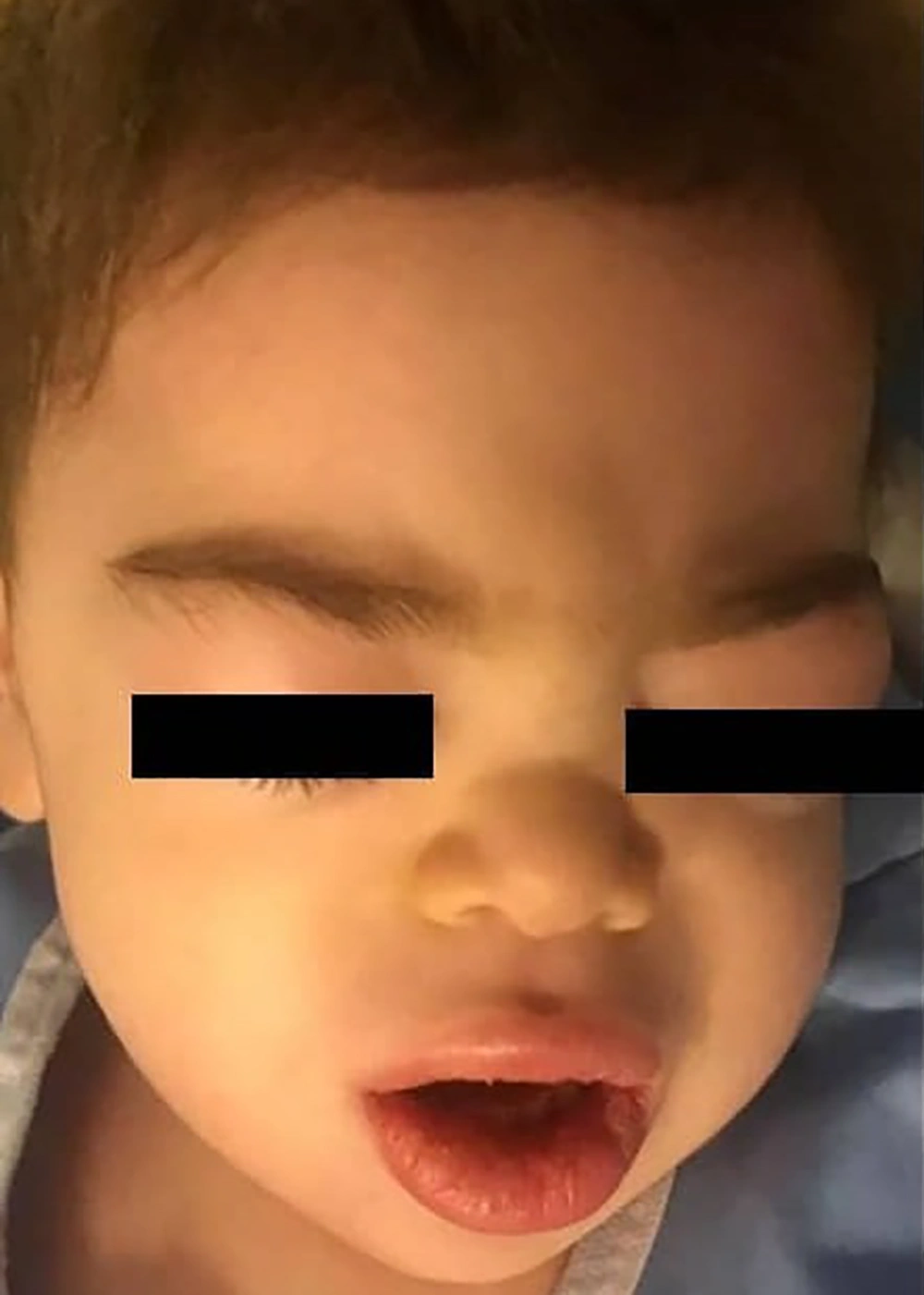
He was lethargic and irritable at the time of admission. Physical examination revealed mild bilateral non-purulent conjunctivitis, mild respiratory distress, and hypoxemia. Laboratory findings revealed absolute lymphopenia, mild thrombocytopenia, increased C-reactive protein (CRP) level, increased erythrocyte sedimentation rate (ESR), and significant elevations in the levels of aspartate transaminase (AST) and alanine transaminase (ALT) (Table 1). Because the child had received high doses of acetaminophen during the previous 3 days, acetaminophen toxicity was initially suspected; however, the serum level of acetaminophen was in the normal range. Kawasaki disease was suspected, but echocardiography revealed a normal left ventricular ejection fraction and no evidence of coronary artery involvement. To confirm COVID-19, chest computed tomography (CT) was performed, and samples from the nasopharynx were subject to real-time polymerase chain reaction (RT-PCR) (9). Findings on chest CT were not suggestive of COVID-19 pneumonitis (Figure 1A). On the second day, the patient’s condition deteriorated. Severe edema developed in the eyelids and lips without peripheral edema; a faint macular erythematous rash appeared over the forehead and back (Figure 2), septic shock ensued, respiratory distress and hypoxemia worsened, and hepatosplenomegaly was confirmed by sonography. Shock was managed according to the guidelines of the American College of Critical Care Medicine (10), with aggressive fluid therapy, epinephrine drip, and empirical antibiotic therapy with meropenem and vancomycin in the pediatric intensive care unit. In addition, a single dose of methylprednisolone (20 mg/kg) was injected to prevent possible severe allergic reactions.
| Day1 | Day2 | Day3 | Day4 | Day6 | Day8 | Day10 | Day14 | Ten Days After Discharge | |
|---|---|---|---|---|---|---|---|---|---|
| pSOFA score | 7 | - | 15 | 7 | 2 | ||||
| Organ systems that failed | Respiratory, coagulation, renal, neurologic | - | Respiratory, coagulation, renal, hepatic cardiovascular neurologic | Respiratory, coagulation, renal, hepatic neurologic | Respiratory renal | ||||
| WBC (reference 3700 - 10000 × 103 cells/µL) | 6000 | 16600 | 12700 | 15600 | 13300 | 8200 | 6600 | 10500 | 13300 |
| Neutrophil, % | 73.8 | 77 | 77.1 | 65.1 | 70.2 | 65 | 69 | 70 | 79.3 |
| Lymphocyte, % | 20.8 | 16.9 | 20.9 | 29.4 | 25.6 | 27 | 27 | 25 | 15.1 |
| Hemoglobin (reference 10. 5 - 13.5 g/dL) | 11.2 | 9.1 | 6.9 | 7.9 | 11.2 | 10.6 | 10.6 | 10 | 10.3 |
| Platelet count (reference 150. 0 - 450.0× 103 cells/µL) | 103000 | 46000 | 23000 | 86000 | 103000 | 119000 | 141000 | 269000 | 306000 |
| AST (reference 15.0 - 42.0 IU/mL) | 499 | 195 | 107 | 53 | 52 | 40 | - | - | 29 |
| ALT (reference 15.0 - 42.0 IU/mL) | 391 | 182 | 129 | 105 | 87 | 63 | - | - | 62 |
| T-bilirubin, mg/dLa | 0. 4 | - | 1. 7 | - | - | 1. 7 | - | 0. 8 | |
| INR | 1. 34 | - | 1. 2 | 2 | > 5 | 2. 7 | 1. 9 | 1 | 1 |
| D-dimer (reference up to 500 ng/mL) | - | - | > 8000 | - | - | > 6000 | - | 4100 | 300 |
| Fibrinogen (reference 200 - 400 mg/dL) | - | - | 101 | - | - | 155 | - | 140 | 147 |
| FDP(reference < 5 µg/mL) | - | - | > 130 | - | - | 25 | - | - | |
| Triglycerides (normal fasting < 265 mg/dL) | - | - | 271 | 277 | - | - | 123 | - | |
| Ferritin (reference 7 - 140 ng/mL) | - | - | 703 | - | - | - | 1650 | 1035 | 199 |
| Lactate dehydrogenase (reference up to 850 IU/L) | - | - | 1036 | - | 1479 | - | 950 | - | 459 |
| C-reactive protein (reference up to 6 mg/L) | 54 | - | - | - | 21 | - | 18 | 11 | |
| ESR 1st h (reference < 15/1st h) | 36 | - | - | - | - | 3 | - | 10 | |
| Procalcitonin, µg/L | 10 | - | - | - | - | - | - | ||
| Creatinin, mg/dLa | 0.7 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aNormal range according Matics and Sanchez-Pinto (11).
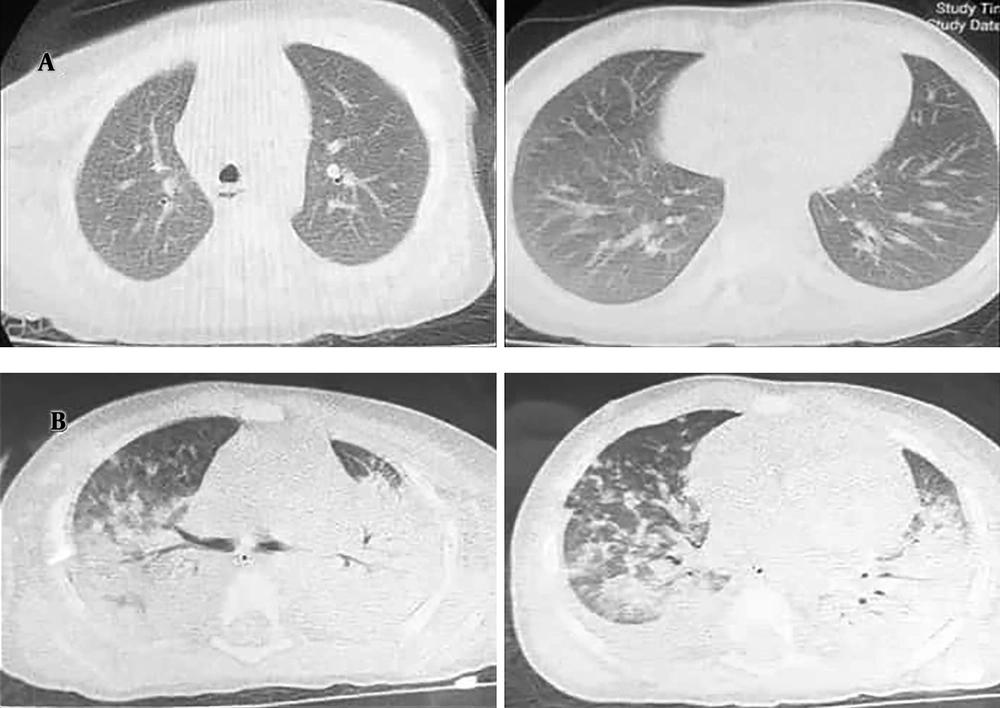
Chest computed tomographic manifestations: Axial slices in an 18-month-old boy with coronavirus disease 19 (COVID-19)-induced secondary hemophagocytic lymphohistiocytosis (sHLH) on the first and third days of hospitalization. A, Scan on the first day of hospitalization, demonstrating no lesions in the lungs; B, scan on the third day of hospitalization, demonstrating bilateral infiltration suggestive of acute respiratory distress syndrome of COVID-19.
On the third day, the patient’s general condition rapidly deteriorated further, with severe respiratory distress and hypoxemia, a decrease in consciousness level, severe irritability, and upper gastrointestinal bleeding. Additional laboratory tests revealed a progressive decrease in platelet counts and hemoglobin level, hypofibrinogenemia, hypertriglyceridemia, and hyperferritinemia (Table 1), which suspected the presence of sHLH. Bone marrow aspiration and biopsy were not done due to the unstable condition of the child and non-adherence of the parents. NK cell activity and soluble CD25 were not evaluated because of insufficient laboratory facilities. The level of interleukin-6 (IL-6) was in the normal range (4.09 pg/mL; normal range: ≤ 7 pg/mL). New chest CT showed peripheral ground-glass opacities and also complete consolidation in the upper and middle thirds of the thorax, which was highly suggestive of ARDS of COVID-19 (Figure 1B). The results of PCR indicated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Treatment with intravenous immunoglobulin (IVIG) (0.5 g/kg/day in four doses) and dexamethasone (10 mg/m2 for two weeks) was initiated. Also, COVID-19 therapy, including hydroxychloroquine and lopinavir/ritonavir, was prescribed. Respiratory support with biphasic positive airway pressure was administered via nasal mask to maintain oxyhemoglobin saturation between 88% and 92% without endotracheal intubation. On the fourth day, because his condition remained serious, a single dose of interferonβ-1a was injected subcutaneously.
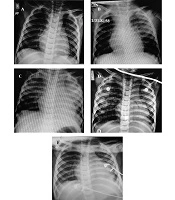
During the following seven days, the clinical and laboratory test results of the patient gradually improved. Fever abated on the fifth day of hospitalization. Irritability decreased, and the level of consciousness continuously improved from the sixth day. Respiratory support was decreased from the fifth day of hospitalization, and epinephrine drip was discontinued on the seventh day. Weaning of non-invasive ventilation (NIV) was initiated from the 7th day of admission, and the child remained in oxygen therapy with mask on the eleventh day and in ambient air from the thirteenth day. Repeated chest radiographs showed a significant decrease in infiltrations in both lung fields (Figure 3). Hepatosplenomegaly improved significantly from the eleventh day on. At discharge on the fourteenth day, the liver was palpable 1 cm below the costal margin, and the spleen was nonpalpable. Laboratory tests showed progressive improvement in platelet count, C-reactive protein level, prothrombin time, international normalized ratio, aspartate transaminase level, and alanine transaminase level, but the ferritin and lactate dehydrogenase levels remained high until discharge. Antiviral and antibiotic therapy was discontinued on the tenth day of hospitalization, and the child was discharged in good condition 14 days after admission with prescription of dexamethasone in tapering doses.
Chest radiographic manifestations: Serial chest radiographs of an 18-month-old boy with coronavirus disease 19 (COVID-19)-induced secondary hemophagocytic lymphohistiocytosis (sHLH). Images on the A, first; B, third; C, fourth; D, sixth; and E, tenth days of hospitalization, showing rapid diminishing of infiltration.
He remained isolated at home for 14 days under close medical observation. Ten days after discharge, he was in good condition without additional symptoms. Laboratory tests revealed a decrease in ferritin and D-dimer without cytopenia, and his recovery was considered successful. Table 1 summarizes the trends in laboratory findings and adapted pediatric Sequential Organ Failure Assessment scores (11).
3. Discussion
We presented the first child with COVID-19-induced HLH and completely recovered with appropriate treatments as a result of early diagnosis. He presented with a high fever, drowsiness, conjunctivitis, and mild respiratory distress, and hypoxemia. His condition progressively deteriorated, met five out of eight criteria of sHLH (5).
To save the life of this patient, in addition to treating COVID-19 according to the national protocol (12), the treatment was based on standard sHLH protocols. The core of HLH treatment is immunosuppressive therapy and chemotherapy. In addition to dexamethasone or etoposide, IVIG is an appropriate supplement in patients with viral infection (5, 6). COVID-19 has imposed a significant medical burden worldwide, and Iran is one of the countries early involved in the pandemic with severe cases (13), even in children (14, 15).
The viral particles of COVID-19, especially in respiratory mucosa, stimulate activation and proliferation of CD8 and natural killer T lymphocytes. The massive release of cytokines by CD8 promotes the multiplication of histiocytes and their differentiation into macrophages, which cross the endothelium to pass into the tissues. Macrophages, in turn, release pro-inflammatory cytokines (IL-1, IL-6, and tumor necrosis factor-α [TNF-α]) that maintain the activation of CD8 T lymphocytes. In this way, a cascade of immune responses is triggered, which leads to cytokine release syndrome and progress to sHLH (16). A recent report in the UK has been noted an unusual presentation in several children similar to atypical Kawasaki disease or Kawasaki disease shock syndrome, but none of them met sHLH criteria (3).
Immunosuppressive agents such as steroids, IVG, and selective cytokine blockade have been suggested to treat these conditions; however, their safety and efficacy have not been verified. COVID-19-induced sHLH has been reported only in two adults with elevated IL-6 levels, which was treated with tocilizumab. Both developed multiorgan failure and died. The patient had received neither the treatment of sHLH nor interferon (8). Because our patient had a normal level of IL-6, tocilizumab was not prescribed.
Type I interferon plays a significant role in antiviral immunity by interfering with viral replication and slowing cell metabolism or secretion of cytokines. Interferon-β is a more potent inhibitor of coronaviruses (17). In China’s national protocol for the treatment of COVID-19 in children, interferon-α nebulization is recommended in severe cases (18). According to the Iranian COVID-19 guideline, interferon β-1a has been suggested for critical COVID-19 cases (13). We decided to administer a single dose of interferon β-1a as an adjuvant therapy with sHLH and antiviral treatment after his clinical condition deteriorated.
Although several forms of COVID-19 treatment are being tested in randomized trials, no drug treatment is currently established. The combination of hydroxychloroquine, lopinavir/ritonavir, and interferon β-1a is being studied in a clinical trial (code: ID IRCT20100228003449N28) (19).
Adverse effects of the MMR vaccine include fever, lymphadenopathy, joint pain, and hypersensitivity reactions; however, sHLH and multiorgan failure have not been previously reported (20). In the present patient, the immune response to the MMR vaccine might lead to an abnormal immune response to the SARS-CoV-2.
The use of interferon β-1a in addition to high-dose dexamethasone and IVIG and antiviral agents coincided with the reversal of our patient’s critical condition. After three days of the treatment, the fever abated, consciousness improved, irritability lessened, the need for respiratory support decreased, and the shock abated. In addition, a decrease in chest radiographic infiltration, reductions in liver and spleen sizes, and improvement in laboratory test results demonstrated that cytokine release and macrophage activation had been well controlled.
The COVID-19 pandemic has caused the deaths of many people, mostly from respiratory manifestations. However, other manifestations and complications such as HLH are life-threatening, that early diagnosis and appropriate treatments can save the patients’ lives.