1. Background
Toxoplasmosis is a zoonotic disease that causes infection worldwide with a prevalence rate of 10 - 80% among the general population in different countries (1, 2). Seroprevalence rates in Iranian general population ranged from 18 to 70% (3). In general, the seroprevalence rate of toxoplasmosis among the Iranian populations has been reported to be 39.3% (4). Overall, the seroprevalence rate of toxoplasmosis among Iranian adolescents aged 10 - 18 years was reported to be 60%, and Toxoplasma gondii IgG and IgM seropositivity was reported to be 56.3 and 3.7%, respectively (5).
The parasite can be transferred by ingestion of food, water, and soil contaminated with cat’s feces containing oocyst, eating raw meat containing tissue cysts, and also through transplacental transmission, which occurs congenitally from mother who acquired her infection during gestation to her child. Thus, caution to decrease the risk of getting active infection should be considered, especially among seronegative pregnant women and immunodeficient patients (6).
Toxoplasmosis is an opportunist infection in immunodeficient individuals, including acquired immune deficiency syndrome (AIDS) patients (7), patients with malignancies, and patients with organ transplants (8). Toxoplasmosis is usually asymptomatic in immunocompetent individuals and may appear as lymphadenopathy and fever that is usually self- limited infection; however, immunocompromised patients are symptomatic, presenting myocarditis, encephalitis, or pneumonitis (9, 10).
Today, due to an increase in the number of organ transplant cases, patients are at risk for opportunistic infections, including toxoplasmosis (11). Solid-organ transplants (SOTs) containing heart, liver, kidney, and bone marrow are at risk of toxoplasmosis (12). Heart transplant recipients are at a higher risk for toxoplasmosis than liver, lung, or kidney transplant recipients (2). The toxoplasmosis that is acquired from the donated organ (13) or reactivation of latent T. gondii cysts may be dormant; however, it is actually active for many years (14). Reactivation risk of toxoplasmosis chronic infection depends on the immunosuppressive therapy and type of organ transplant (15). The risk of acquiring toxoplasmosis infection in a seronegative recipient from a seropositive donor is highest in heart transplant recipients among SOT recipients (11). Definitive diagnosis of toxoplasmosis is according to the presence of parasites or parasitic DNA in blood, biological fluids, or biopsy specimens, and serological tests are not enough alone; however, serological tests of donors and recipients before transplantation can help to identify seronegative recipients who are at risk for toxoplasmosis when receiving an organ from a seropositive donor (16).
Chemoprophylaxis has improved the consequence of toxoplasmosis for SOT recipients. Standardized guidelines are needed for prophylactic regimens and patient management (9). The efficacy of trimethoprim-sulfamethoxazole (cotrimoxazole) in the prevention of toxoplasmosis after hematopoietic cell transplant (HCT) and for SOT recipients has been reported (15, 16). Furthermore, blood polymerase chain reaction (PCR) is a useful method for the diagnosis of the infection earlier. PCR targeting the B1 gene with the use of primers, such as B22 and B23 because of their high sensitivity and specificity was recommended for diagnosing toxoplasmosis in previous studies. Also, T. gondii bradyzoite-specific genes (SAG-4, MAG-1) had good performance for diagnosing toxoplasmosis, especially after prophylaxis or treatment (17-19).
2. Objectives
The objective of this study was to evaluate toxoplasmosis by PCR using stage-specific oligonucleotide primers for the diagnosis of toxoplasmosis in pediatric heart transplant recipients who were under cotrimoxazole as prophylaxis regimens at Rajaei Cardiovascular and Medical Research Center, Iran University of medical sciences, Tehran, Iran.
3. Methods
In this study, blood samples were collected from 46 pediatric heart transplant recipients who underwent cardiac transplantation during 2018 - 2019 at Rajaei Heart Hospital, Tehran. All the cases were on long-life oral administration of cotrimoxazole. All patients were under immunosuppressive treatment with mycophenolate, tacrolimus, and prednisolone.
The blood samples were used for peripheral blood mononuclear cell (PBMC) preparation and also for detection of specific Toxoplasma IgM and IgG antibodies. The PBMC isolation was performed from blood collected in tubes containing ethylenedinitrilotetraacetic acid (EDTA) using Ficoll, according to the previous study (7).
3.1. DNA Extraction and PCR
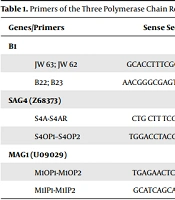
The DNA was extracted from the patients’ blood (PBMC) using the QIAamp DNA mini kit (Qiagen, Hilden, Germany). The samples were evaluated with B1 and bradyzoite-specific genes (SAG-4 and MAG-1) for toxoplasmosis infection or reactivation. The primers were selected within the B1 gene of 115 -bp fragment as previously described (18, 20). Sequence primer pairs were employed for SAG-4, and nested PCR was performed with amplification of a 126-bp PCR product (21). The oligonucleotide was used for MAG-1, which resulted in PCR product sizes of 212 bp (19). The PCR protocol was performed according to the conditions previously described (17, 19). Sequence primer pairs employed in nested PCR are summarized in Table 1. All PCR reactions were performed with positive control, that was DNA from T. gondii (RH strain), and distilled water was used as a negative control.
| Genes/Primers | Sense Sequence 5’ – 3’ | Antisense Sequence 5’ – 3’ | Band (bp) | Ref |
|---|---|---|---|---|
| B1 | ||||
| JW 63; JW 62 | GCACCTTTCGGACCTCAACAACCG | TTCTCGCCTCATTTCTGGGTCTAC | 286 | Okay et al., 2009 (18) |
| B22; B23 | AACGGGCGAGTAGCACCTGAGGAGA | TGGGTCTACGTCGATGGCATGACAAC | 115 | Bretagne et al., 1993 (20) |
| SAG4 (Z68373) | Odberg-Ferragut et al., 1996 (21) | |||
| S4A-S4AR | CTG CTT TCG TCT GTC TTCAAC | CTT CTT CAC TGG CAA TGAACT C | 562 | |
| S4OP1–S4OP2 | TGGACCTACGATTTCAAGAAGGC | GCTGCGAGCTCGACGGGCTCATC | 187 | |
| MAG1 (U09029) | Contini et al., 2002 (19) | |||
| M1OP1-M1OP2 | TGAGAACTCAGAGGA CGTTGC | TCT GAC TCAAGC TCG TCTGCT | 518 | |
| M1IP1-M1IP2 | GCATCAGCATGAGACAGAAGA | CCAACTTCGAAACTGATGTCG | 212 |
Primers of the Three Polymerase Chain Reaction (PCR) Systems Used in the Present Study
3.2. Enzyme-Linked Immunosorbent Assay
In this study, 46 pediatric heart transplant recipients were evaluated for IgG and IgM T. gondii antibodies using enzyme-linked immunosorbent assay (ELISA) (Uroimmun, Germany) according to the manufacturer’s instruction. For IgG, positive results were > 11 IU/mL, while IgG negative and borderline results were < 8 IU/mL and 8 - 11 IU/mL, respectively. For IgM, positive results were > 1.1 IU/mL, while negative and borderline results were < 0.8 IU/mL and 0.8 - 1.1 IU/mL, respectively.
4. Results
The age range of pediatric heart transplants was 1 - 17 years, with a mean age of 10.18 ± 4.01 years. Of the 46 patients, 18 patients were female, and 28 were male. The most common disorders were dilated cardiomyopathy (DCM) (94.8%), restrictive cardiomyopathy (RCM) (2.6%), and RCM + hypertrophic cardiomyopathy (HCM) (2.6%). No toxoplasmosis symptoms were found in heart transplant recipients.
4.1. ELISA Results
All 46 patients were evaluated for T. gondii-specific IgM antibody before and after transplantation, and all were negative for T. gondii IgM antibody before and after transplantation. Furthermore, 6 patients (13.05%) were positive for IgG T. gondii antibody, while 40 cases (86.95%) were negative for IgG before transplantation. In addition, 3 (6.5 %) out of 46 patients were positive for T. gondii IgG antibody, and 43 cases (93.5%) patients were negative for anti-T. gondii IgG antibody after transplanation. Seroconversion from negative to positive T. gondii IgG antibody before and after transplantation was not found (Table 2).
| Pediatric Transplant Recipients | Toxoplasma gondii IgG Antibody Before Transplant | Toxoplasma gondii IgM Antibody Before Transplant | Polymerase Chain Reaction After Transplant | Toxoplasma gondii IgG Antibody After Transplant | Toxoplasma gondii IgM Antibody After Transplant |
|---|---|---|---|---|---|
| Positive | 6 (13.05) | 0 | 0 | 3 (6.5) | 0 |
| Negative | 40 (86.95) | 46 (100) | 46 (100) | 43 (93.5) | 46 (100) |
| Total | 46 | 46 | 46 | 46 | 46 |
Toxoplasma gondii IgG and IgM Antibodies in Pediatric Transplant Recipients
4.2. PCR Results
All 46 patients were evaluated using PCR with B1 and bradyzoite-specific genes (SAG-4 and MAG-1) genes for infection and reactivation in this study. The results of PCR using B1 and bradyzoite-specific genes (SAG-4, MAG-1) genes were negative for all 46 heart transplant recipients. In each set of PCR, the results of positive (Toxoplasma RH strain) and negative controls were confirmed.
5. Discussion
T. gondii primary infection/reactivation after SOT is a serious problem, because of the high mortality rate after disseminated disease. Toxoplasmosis frequency in heart transplant recipients was higher in comparison with the kidney and liver transplant ones (22). In this study, 46 pediatric heart transplant recipients were evaluated for the toxoplasmosis. The results showed that all 46 patients had negative T. gondii IgM antibody and also 46 patients were evaluated for T. gondii specific IgG antibody and 3 (6.5 %) out of 46 patients had positive T. gondii IgG antibody and 43 (93.5%) patients had negative T. gondii IgG antibody after transplant. It should be noted that negative results of serological testing are one of the risk factors for increased mortality due to Toxoplasma infection in heart transplant recipients that was previously described (23).
In the present study, 6 patients (13.05%) were positive for T. gondii IgG antibodies before transplant, of whom 3 cases were IgG negative after transplant that these results may be due to using immunosuppressive therapy in transplant patients, which has been previously reported (16, 24, 25). In a study, Toxoplasma IgG titration was decreased in a heart transplant recipient following 6 months due to a decrease in antibody production caused by immunosuppression (24). In another study, the mean IgG titer after transplant of patients who were IgG positive before transplant significantly decreased from 20.12 units to 2.2 ± 1.93 IU/mL. This seroreversion could be related to immunosuppressive therapy that was received by the patients. Furthermore, it was indicated that the mean Toxoplasma IgG titration after transplant decreased after the first 6 months in comparison with more than 12 months after transplant (25).
The results of previous studies have indicated that PCR using specific primers for the SAG-4 and MAG-1 genes is efficient in diagnosing toxoplasmosis, especially in patients who were under prophylaxis regimen or at the onset of treatment (17, 19). Furthermore, nested PCR using primers B22/B23 was reported as a useful method for diagnosing toxoplasmosis (18). In the present study, for the first time, PCR using B1 and bradyzoite-specific genes (SAG-4 and MAG-1) was used to evaluate Toxoplasma infection/reactivation in pediatric heart transplant patients in Iran. The results of PCR were negative for all 46 heart transplant recipients. Neither toxoplasmosis infection nor seroconversion from negative to positive T. gondii IgG antibody before and after transplantation occurred in patients who received cotrimoxazole. None of the patients had any symptoms of toxoplasmosis. The findings of this study are compatible with previous studies indicating that cotrimoxazole can be effective in preventing toxoplasmosis in heart transplant patients.
The importance of using cotrimoxazole prophylaxis during the post-transplant period in the prevention of toxoplasmosis has been reported in some previous studies (26-28). In a study, 417 orthotopic cardiac transplant patients received post-transplant cotrimoxazole during 8 months and were evaluated for toxoplasmosis, and acute toxoplasmosis was observed in a patient after transplantation (0.2%). The results indicated that cotrimoxazole prophylaxis is effective after orthotopic cardiac transplantation (26). In a study, 315 patients underwent heart transplantation in Spain, and Toxoplasma IgG antibody serostatus of 32 patients (10.2%) was donors+/recipients-; 12 patients received pyrimethamine, 17 patients received cotrimoxazole, and 3 patients did not receive prophylaxis. Toxoplasmosis was observed in two patients who did not receive any kind of prophylaxis, and finally, these two patients were treated with pyrimethamine and sulfadiazine with satisfactory results. Seroconversion without clinical manifestation occurred in one patient who received pyrimethamine. Neither seroconversion nor toxoplasmosis occurred in patients who received cotrimoxazole (27).
A retrospective study was performed for the assessment of toxoplasmosis in 436 adult Brazilian heart transplant recipients. The results indicated that six patients (1.3%) developed T. gondii infection/reactivation in the post-operative period, and three patients (50%) died due to toxoplasmosis. All infections/reactivations occurred when no cotrimoxazole prophylaxis or prophylactic regimen was used (29).
In a review of two transplant programs, no evidence of toxoplasmosis was found after heart transplantation because of prophylaxis for Pneumocystis jiroveci with cotrimoxazole. These programs were efficient for the prevention of Toxoplasma infection or reactivation (30). Toxoplasmosis is one of the most serious opportunistic infections that may be occurred after transplantation and can be dangerous in cases of delayed diagnosis. It should be noted that the infection is more observed in patients without receiving prophylaxis. In general, because of the negative results of Toxoplasma with PCR using B1 and bradyzoite-specific genes (MAG-1 and SAG-4) it is possible that the results obtained in this study are due to prophylaxis with cotrimoxazole.
5.1. Conclusion
In this study, due to the negative results of PCR and the effectiveness of cotrimoxazole in preventing toxoplasmosis in other studies, prophylaxis with cotrimoxazole may be successful in preventing toxoplasmosis in the heart transplant patients.