1. Background
Streptococcus pneumoniae is known as a gram-positive diplococcus inducing several invasive and non-invasive infections, including pneumonia, sepsis, meningitis, sinusitis, and acute otitis media (AOM) in children, resulting in high morbidity and mortality rates, especially in individuals aged below five years (1, 2).
Streptococcus pneumoniae can be colonized in the upper airway, and the human is the only natural reservoir of this microorganism (3). The carriers of S. pneumoniae might be asymptomatic (4). Pharyngeal colonization with S. pneumoniae may also occur at any age, and the first colonization is usually detected at the age of 4 - 6 months (5, 6). Typically, there is a relationship between age, socioeconomic status, and attendance in daycare centers with the colonization risk and rate induced by this microorganism (7).
The prevalence rate of pneumococcal colonization in children aged below five years varies from 2 - 93.4% (6-10).
In India and Bangladesh, colonization induced by this microorganism starts at 2 - 3 months of age, and 80% of children are colonized by the age of 6 months. In developed countries such as the United States, the colonization rate starts and increases dramatically after six months of age due to the more frequent attendance of infants in daycare centers (9).
In this regard, previous studies have demonstrated that attendance in daycare centers and family size could be remarkable risk factors of colonization (11). However, factors converting colonization to diseases are not precisely defined. Progression to invasive diseases is more likely in young children, older adults, and patients with comorbidities (12). A higher pneumococcal density is likely to facilitate transmission and microaspiration to the lungs, thereby increasing the likelihood of progression to diseases (12).
Breastfeeding has a protective effect due to the provision of many ingredients, including immunoglobulin A (IgA), lactoferrin, and oligosaccharides, which act as analog receptors providing secondary protection against bacteria colonization in the nasopharynx (13, 14). Nevertheless, some studies have not explicitly confirmed the protective role of breastfeeding in preventing colonization (13, 15).
The carriage evaluation is of paramount importance because colonization can lead to invasion and diseases (12). Currently, Iran has excluded pneumococcal vaccine in the Expanded Program on Immunization (EPI), and it is only recommended to high-risk individuals. Moreover, it is
administered on-demand in private sectors for children aged below five years (16). This study was to increase information on circulating serotypes in Iran at different ages. Furthermore, the study aimed to determine the coverage of the existing PCV13 regarding the most common circulating serotypes in carriers.
The study findings would contribute to determining the circulating serotypes of S. pneumoniae in infants in Iran and facilitate making decisions to select appropriate vaccines. Moreover, this study was conducted in the pre-vaccine phase, and the results can be compared with those obtained for the post-vaccination phase in the future to estimate the potential impact of the vaccine on the circulating S. pneumoniae serotypes in Iran and monitor pneumococcal dynamics in vaccinated subjects.
2. Objectives
This study aimed to evaluate the serotype distribution of S. pneumoniae carriage in six-month-old infants.
3. Methods
3.1. Sample Size and Study Design
This descriptive cross-sectional study was included 600 six-month-old infants and conducted from October 2017 to 2018 after obtaining the approval of the Ethics Committee of the Iran University of Medical Sciences (Code: IR.IUMS.REC.1395.9311165006). The infants’ parents or guardians signed written consent forms before the study.
Following physical examinations, the healthy infants aged six months referred to public health centers for vaccine administration were included in the study. Exclusion criteria were infectious diseases at the sampling time, antibiotic consumption over the last two weeks, underlying disorders, and congenital or acquired immunodeficiency disorders. Finally, a pediatric resident completed a questionnaire for each enrolled case.
3.2. Sampling and Streptococcus pneumoniae Detection
To this end, a pre-vaccination pharyngeal sample was taken from each case with a Dacron swab in the healthcare centers by a trained pediatric resident and transferred to the Laboratory of Pediatric Infections Research Center (PIRC), Mofid children's Hospital, Tehran, Iran. triple sugar iron (TSI) culture medium was meant.
The swabs were immediately transported to the laboratory and then processed. Afterwards, they were cultured in the laboratory on a chocolate agar medium and incubated with a 10% CO2 incubator. After 48 hours, the grown isolates suspected of S. pneumoniae were examined by the following methods. After 48 hours, the concerned isolates were examined using specific microbiology and biochemical assays such as Catalysis, gram stain, optochin sensitivity, and alpha hemolysis on bloody agar plates. Optochin sensitivity and bile solubility tests were performed to isolate S. pneumoniae from other streptococci such as S. viridans (17, 18).
3.3. Molecular Confirmation of Streptococcus pneumoniae
DNA was extracted by High Pure PCR Template Preparation Kit (Roche, product No. 11796828001) and kept at -80°C.
Moreover, S. pneumoniae was confirmed by the capsular polysaccharide (CPS) proliferation with PCR. The forward and reverse primer amplified the 160bp nucleotide fragment using 5’GCAGTACAGCAGTTTGTTGAACTGACC3’ and 5’GAATATTTT CATT ATC AG TC C- CAGTC3’sequences, respectively (19).
3.4. Serotyping pneumococcus with Multiplex PCR
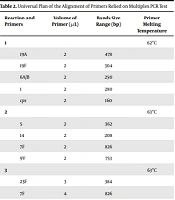
Twenty- five different serotypes of the confirmed S. pneumoniae were prepared by multiplex PCR. Table 1 presents the primers, and Table 2 shows different multiplex reaction groups (20).
| Primers | Primer Sequence 5′ to 3′ | Bands Size Range (bp) |
|---|---|---|
| 1 | F: CTC TAT AGA ATG GAG TAT ATA AAC TAT GGT TA | 280 |
| R: CCA AAG AAA ATA CTA ACA TTA TCA CAA TAT TGG C | ||
| 4 | F: CTG TTA CTT GTT CTG GAC TCT CGA TAA TTG G | 430 |
| R: GCC CAC TCC TGT TAA AAT CCT ACC CGC ATT G | ||
| 3 | F: ATG GTG TGA TTT CTC CTA GAT TGG AAA GTA G | 371 |
| R: CTT CTC CAA TTG CTT ACC AAG TGC AAT AAC G | ||
| 5 | F: ATA CCT ACA CAA CTT CTG ATT ATG CCT TTG TG | 362 |
| R: GCT CGA TAA ACA TAA TCA ATA TTT GAA AAA GTA TG | ||
| 6A/B | F: AAT TTG TAT TTT ATT CAT GCC TAT ATC TGG | 250 |
| R: TTA GCG GAG ATA ATT TAA AAT GAT GAC TA | ||
| 7F | F: CCT ACG GGA GGA TAT AAA ATT ATT TTT GAG | 826 |
| R: CAA ATA CAC CAC TAT AGG CTG TTG AGA CTA AC | ||
| 7C | F: CTA TCT CAG TCA TCT ATT GTT AAA GTT TAC GAC GGG A | 260 |
| R: GAA CAT AGA TGT TGA GAC ATC TTT TGT AAT TTC | ||
| 8 | F: GAT GCC ATG AAT CAA GCA GTG GCT ATA AAT C | 294 |
| R: ATC CTC GTG TAT AAT TTC AGG TAT GCC ACC | ||
| 9V | F: CTT CGT TAG TTA AAA TTC TAA ATT TTT CTA AG | 753 |
| R: GTC CCA ATA CCA GTC CTT GCA ACA CAA G | ||
| 10A | F: GGT GTA GAT TTA CCA TTA GTG TCG GCA GAC | 628 |
| R: GAA TTT CTT CTT TAA GAT TCG GAT ATT TCT C | ||
| 11A | F: GGA CAT GTT CAG GTG ATT TCC CAA TAT AGT G | 463 |
| R: GAT TAT GAG TGT AAT TTA TTC CAA CTT CTC CC | ||
| 12F | F: GCA ACA AAC GGC GTG AAA GTA GTT G | 376 |
| R: CAA GAT GAA TAT CAC TAC CAA TAA CAA AAC | ||
| 14 | F: CTT GGC GCA GGT GTC AGA ATT CCC TCT AC | 208 |
| R: GCC AAA ATA CTG ACA AAG CTA GAA TAT AGC C | ||
| 15B | F: ATT AGT ACA GCT GCT GGA ATA TCT CTT C | 436 |
| R: GAT CTA GTG AAC GTA CTA TTC CAA AC | ||
| 16F | F: TTG GAA TTT TTT AAT TAG TGG CTT ACC TA | 988 |
| R: CAT CCG CTT ATT AAT TGA AGT AAT CTG AAC C | ||
| 17F | F: TTC GTG ATG ATA ATT CCA ATG ATC AAA CAA GAG | 693 |
| R: GAT GTA ACA AAT TTG TAG CGA CTA AGG TCT GC | ||
| 18C-F | F: CTT AAT AGC TCT CAT TAT TCT TTT TTT AAG CC | 573 |
| R: TTA TCT GTA AAC CAT ATC AGC ATC TGA AAC | ||
| 19A-F | F: GTT AGT CCT GTT TTA GAT TTA TTT GGT GAT GT | 478 |
| R: GAG CAG TCA ATA AGA TGA GAC GAT AGT TAG | ||
| 19F | F: GTT AAG ATT GCT GAT CGA TTA ATT GAT ATC C | 304 |
| R: GTA ATA TGT CTT TAG GGC GTT TAT GGC GAT AG | ||
| 20 | F: GAG CAA GAG TTT TTC ACC TGA CAG CGA GAA G | 514 |
| R: CTA AAT TCC TGT AAT TTA GCT AAA ACT CTT ATC | ||
| 23F | F: GTA ACA GTT GCT GTA GAG GGA ATT GGC TTT TC | 384 |
| R: CAC AAC ACC TAA CAC ACG ATG GCT ATA TGA TTC | ||
| 31 | F: GGA AGT TTT CAA GGA TAT GAT AGT GGT GGTGC | 701 |
| R: CCG AAT AAT ATA TTC AAT ATA TTC CTA CTC | ||
| 34 | F: GCT TTT GTA AGA GGA GAT TAT TTT CAC CCA AC | 408 |
| R: CAA TCC GAC TAA GTC TTC AGT AAA AAA CTT TAC | ||
| 35B | F: GAT AAG TCT GTT GTG GAG ACT TAA AAA GAA TG | 677 |
| R: CTT TCC AGA TAA TTA CAG GTA TTC CTG AAG CAA G | ||
| 35F | F: GAA CAT AGT CGC TAT TGT ATT TTA TTT AAA GCA A | 517 |
| R: GAC TAG GAG CAT TAT TCC TAG AGC GAG TAA ACC |
Arrangement of Primers to Serotype from Pneumococcus Strains Applying Multiplex PCR
| Reaction and Primers | Volume of Primer (µL) | Bands Size Range (bp) | Primer Melting Temperature |
|---|---|---|---|
| 1 | 62°C | ||
| 19A | 2 | 478 | |
| 19F | 2 | 304 | |
| 6A/B | 2 | 250 | |
| 1 | 2 | 280 | |
| cps | 2 | 160 | |
| 2 | 63°C | ||
| 5 | 2 | 362 | |
| 14 | 2 | 208 | |
| 7F | 2 | 826 | |
| 9V | 2 | 753 | |
| 3 | 63°C | ||
| 23F | 3 | 384 | |
| 7F | 4 | 826 | |
| 11A | 2 | 463 | |
| 1 | 2 | 280 | |
| cps | 2 | 160 | |
| 4 | 62°C | ||
| 16F | 4 | 988 | |
| 18C | 2.5 | 573 | |
| 35B | 2 | 677 | |
| 12F | 2 | 376 | |
| 5 | 61°C | ||
| 8 | 3 | 294 | |
| 3 | 3 | 371 | |
| 15B | 3 | 496 | |
| 31 | 4 | 701 | |
| 6 | 60°C | ||
| 1 | 3 | 280 | |
| 10A | 3 | 628 | |
| 35F | 3 | 517 | |
| 34 | 4 | 408 | |
| 7 | 63°C | ||
| 20 | 2 | 514 | |
| 7C | 2 | 260 | |
| 17F | 2 | 693 | |
| 4A | 2 | 430 |
Universal Plan of the Alignment of Primers Relied on Multiplex PCR Test
3.5. Statistical Analysis
The quantitative data in this study were expressed as mean ± standard deviation (SD); however, the qualitative ones were described as percentages. T-test and Mann-Whitney U test were used to compare quantitative data with normal and non-normal distribution, respectively. The qualitative variables with normal and non-normal distribution were also compared using the Chi-square test or Fisher’s exact test, respectively. Pearson correlation coefficient and Spearman rank-order correlation were also used to examine the relationship among the quantitative variables. Moreover, the multivariate logistic regression analysis determined the differences in the indices in the presence of the basic features. The results were also presented as odds ratio (OR) (95% confidence interval: CI). The IBM SPSS software version 21 was used for the statistical analysis of the data, and the significance level was set as P < 0.05.
4. Results
Table 3 presents the participants’ demographic information in the two breastfed and formula-fed groups. As shown in this table, none of the cases in this study had received pneumococcal vaccines or been kept in daycare centers. Of 600 infants, 13 cases (2%) (namely seven formula-fed and six breastfed infants) had positive S. pneumoniae culture with no significant difference between the two groups (P = 0.8). The characteristics of the two culture-positive and culture-negative groups are outlined in Table 4.
| Characteristics | Breast fed (n = 300) | Formula fed (n = 300) | Total (n = 600) | P-Value |
|---|---|---|---|---|
| Gender (male) | 134 (46.5) | 154 (53.5) | 289 (48.16) | 0.1 |
| Hospital admission duration (days) | 7.4 ± 4.03 | 11.08 ± 7.98 | 0.3 | |
| History of antibiotic consumption during the last six months | 14 (4.7) | 42 (14) | 56 (9.33) | 0.001 |
| Positive history of URI | 14 (4.7) | 37 (3/12.3) | 51 (8.5) | 0.001 |
| Positive history of URI in siblings | 67 (22.4) | 58 (19.4) | 125 (20.83) | 0.2 |
| Prematurity | 26 (8.79) | 51 (17.1) | 77 (12.83) | 0.002 |
| Normal vaginal delivery (NVD) | 160 (54.2) | 117 (49.2) | 277 (46.16) | 0.2 |
| Nationality | 0.04 | |||
| Iran | 269(89.7) | 283 (94.3) | 552 (92) | |
| Afghanistan | 31 (10.3) | 17(5.7) | 48 (8) | |
| Smoker parents | 103 (34.7) | 114(38.4) | 217 (36.16) | 0.3 |
| Pharyngeal pneumococcal carriage | 6 (%2) | 7(2.33) | 13 (2.16) | 0.8 |
| Hospitalization (No. of episodes) | 54 (18) | 68(22.7) | 122 (20.33) | 0.1 |
| Admission cause | ||||
| Pneumonia | 13 (4.3) | 6 (2) | 19 (3.16) | 0.1 |
| Diarrhea | 9 (3) | 9 (3) | 18 (3) | 1.000 |
| Uti | 3 (1) | 0 | 3 (0.5) | 0.2 |
| Bronchiolitis | 14 (4.6) | 37 (12.3) | 51 (8.5) | 0.001 |
| Total | 300 | 300 | 600 |
Clinical Characteristics of Breastfed and Formula-Fed Infants
| Culture Positive (n = 13) | Culture Negative (n = 587) | P-Value | |
|---|---|---|---|
| Feeding | 0.8 | ||
| Breast | 6 (2) | 294 (98) | |
| Formula | 7 (53.86) | 293 (49.9) | |
| History of hospital admission | 3 | 0 | < 0.00001 |
| Vaginal delivery | 8 (61.5) | 291 (49.57) | 0.4 |
| Nationality | 0.001 | ||
| Iranian | 11 (84.6) | 541 (92.16) | |
| Afghanistan | 2 (15.4) | 46 (7.84) | |
| URI in family at sampling time | 0.001 | ||
| Breast | 2(33%) | 94(16) | |
| Formula | 6(85) | 123(21) | 0.00001 |
| Sibling in daycare centers | 11 | 0 |
Clinical Characteristics of Colonized (Pharyngeal Culture-Positive) vs. Non-colonized Infants
Out of 13 culture-positive cases, seven infants were formula-fed, three infants had a history of hospitalization (namely one case of gastroenteritis (GE) and two cases of pneumonia), and none of the six culture-positive cases in the breastfed group had a hospitalization history.
Table 5 presents the S. pneumoniae serotypes isolated from the pharynx of the infants.
| Pneumococcal Serotypes | Breastfed | Formula-Fed | Total |
|---|---|---|---|
| 1 | - | - | 0 |
| 3 | 3 | 0 | 3 |
| 4 | - | - | 0 |
| 5 | - | - | 0 |
| 6A | 1 | - | 1 |
| 7F | - | 1 | 1 |
| 7C | - | - | 0 |
| 8 | - | - | 0 |
| 9V | - | - | 0 |
| 10A | - | - | 0 |
| 11A | 1 | - | 1 |
| 12 | - | - | 0 |
| 14 | - | 1 | 1 |
| 15A | - | - | 0 |
| 15B | 1 | - | 1 |
| 16 | - | - | 0 |
| 17 | - | - | 0 |
| 18C | - | - | 0 |
| 19F | 1 | - | 1 |
| 19A | 1 | - | 1 |
| 20 | - | - | 0 |
| 22F | - | - | 0 |
| 23F | 1 | 5 | 6 |
| 31 | - | - | 0 |
| 33F | - | - | 0 |
| 34 | 1 | - | 1 |
| 35B | - | - | 0 |
| 35F | - | - | 0 |
| 38 | - | - | 0 |
| 6C | - | - | 0 |
| 23A | 1 | - | 1 |
| 23B | - | - | 0 |
| Total | 11 | 7 | 18 |
Comparison of Pneumococcal Serotypes Isolated from Breast and Formula-Fed Infants
The most frequent serotype in formula-fed infants was Serotype 23F (n = 5, 1.7%); however, serotype 3 (n = 3, 1%) in the breastfed group was the most frequent one. Interestingly, co-colonization phenomena were observed in three breastfed and two formula-fed infants. Moreover, the association of 19F /23 F and 7F/11A/23F was noticed in the formula-fed group, and the co-colonization of 6A/34, 3/15B, and 3/23A was observed in the breastfed group.
In general, Serotype 23F (1%) was the most common isolated serotype. Accordingly, PCV13, PCV10, and PPSV23 pneumococcal vaccines had 73%, 50%, and 84% stereotype-specific coverage, respectively. In the subgroup analyses, stereotype-specific pneumococcal vaccine coverage rates in breastfed and formula-fed infants were 30%, 18%, 100%, and 62%, 100%, 66% for PCV13, PCV10, and PPSV23, respectively.
5. Discussion
To the best of the authors’ knowledge, this study was the first attempt in Iran to evaluate the S. pneumoniae colonization in six-month-old infants, exactly before starting supplementary food.
The colonization rate of S. pneumoniae was 2% in this study. In contrast, an investigation in Gambia in 2006 (10) reported a higher colonization rate of 97% in infants aged below one year. It should be noted that the colonization rate is dependent on socioeconomic status, environmental and host factors, age, and study settings.
Low socioeconomic status and environmental factors (e.g., daycare attendance, living in a family with other young children) are risk factors increasing the likelihood of pneumococcal carriage (21, 22).
In a study in Mashhad, Iran, on children aged 2-6 years, the colonization rate was 13.1% (15). In this regard, the colonization rate seems to increase with age in childhood as such, the participants' age was one of the main reasons for lower colonization rate in this study compared to other studies conducted in Iran. Furthermore, the colonization rate in different parts of the body may also differ.
In the present study, 2% of the participants (13 out of 600 infants) (namely seven formula-fed and six breastfed cases) were positive for S. pneumoniae, revealing no significant difference between the two groups. Although the protective role of breast milk in preventing infections has been documented, the colonization rates were not significantly different between the two groups. In this regard, a trial study was carried out, and a strong association was observed between breastfeeding and microbial community composition in the upper respiratory tract of six-week-old infants, which may contribute to the protective effect of breastfeeding on respiratory infections in the early infancy (23). Interestingly, the relationship between breastfeeding and nasopharyngeal microbiota composition disappeared in the six-month-old infants. Although the sample size was small in the present study, which might have affected the results, the non- significant difference between these two groups might be due to the participants’ age and, consequently, the decreased effect of breast milk on colonization rates in infants aged six months.
In the present study, there was no significant difference between the colonization rates of S. pneumoniae in the two groups. In a study in Iran, no significant difference was observed between the breastfed and formula-fed cases. However, their study was included children aged 2-6-year-old. The findings might have been affected by several factors and several intervening variables (15).
The findings reported in the United States in 1993 were in concordance with those of the present study (24). Accordingly, the researchers concluded that exclusive breastfeeding could not significantly induce colonization with common bacterial respiratory pathogens two months after birth (24).
In our study, prematurity was noticed in 8.79% of breastfed infants and in about half (17.3%) of the formula-fed participants (P = 0.002). This difference should be evaluated carefully because most premature infants can not be fed by their mothers, and there are confounding factors regarding this statistical difference.
Regardless of the type of feeding, Serotype 23F was the most frequent serotype isolated in the present study. No similar study in Iran has compared 6-month-old infants to reach the same finding. However, a study in Taiwan demonstrated that serotypes 23F, 6B, 19F, and 14 were the most frequent colonizing ones (25). Interestingly, a systematic review evaluating the distribution of S. pneumoniae serotypes in carriers and patients in Iran introduced Serotype 23F as the most frequent serotype inducing invasive pneumococcal diseases (16). The similarity between the most frequent colonizing serotypes in this study and those inducing diseases in a recent systematic review may indicate that pneumococcal pharyngeal carriage is a prerequisite for the development of invasive pneumococcal diseases (26). In this regard, the most frequent serotype in formula-fed infants was Serotype 23F; however, the most frequent serotype was Serotype 3 in the breastfed participants. Although there was no difference between the two groups regarding the frequency of pneumococcal carriage, the type of feeding could affect the pneumococcal serotypes colonizing the infants. In this regard, Serotype 23F is included in all existing pneumococcal vaccines, including conjugate (7-,10- and 13- valent) and 23-valent polysaccharide vaccines.
In this study, some formula-fed and breastfed infants were involved in co-colonization. Some researchers have reported the association between co-colonization an acute respiratory infection. The interactions of multiple serotypes and their role in increasing the microorganism pathogenicity have been suggested; However, co-colonization may yield to growing competition among the serotypes, which controls their overall growth rate and pathogenicity. In other words, the main role of co-colonization remains to be defined in the future (27).
In the present study, 11 out of 13 infants colonized with S. pneumoniae had siblings referring to daycare centers and kindergartens, and the value was statistically significant. This finding implies that attendance in such centers and having a sibling referring to such places can be risk factors for the S. pneumoniae colonization.
The small sample size was a limitation of this study. Limited number of age groups and the low carriage rate at this age resulted in the low prevalence of positive cases. Future studies are suggested to include larger sample sizes or more age groups. The studies can also focus on risk factors, vaccination coverage, or cohort studies to evaluate pathogenicity.
To sum up, in infants aged six months, the most common isolated S. pneumoniae serotype was serotyped 23, and PCV13 had a 73% coverage on the isolated serotypes in this study. The study findings, however, fail to confirm the effectiveness of early 23-valent polysaccharide vaccination in the general infant population or those with risk factors (i.e., infants or those with siblings referring to daycare centers). Considering the implicit and explicit costs, cost-effectiveness studies are suggested to evaluate the effectiveness of this early vaccination and the potential harms of its ignorance.
5.1. Conclusions
In conclusion, in infants aged six months, the most common isolated S. pneumoniae serotype was Serotypes 23, and PCV13 had a 73% coverage on the isolated serotypes in this study.
Studies with larger sample sizes or different age groups are recommended to evaluate the potential risk factors and the efficacy of early immunization interventions.