1. Background
According to the Ministry of Health and Family Welfare, Government of India, 7,597,063 cases of coronavirus disease 2019 (COVID-19) and 115,197 deaths have been reported until October 2020 in India (1). The major source for the spread of this virus is the respiratory droplets and/or close contact with infected people and family clustering (2). Older people, individuals with comorbidities, and pregnant women are more prone to this virus which causes adverse outcomes and mortality (3). Pregnant women are highly susceptible due to their physiological and immunological changes (4).
Recent systematic reviews and meta-analyses described the various characteristics of COVID-19 positive mothers and their neonates (5-7). Independent prospective studies, case series, and case reports have yielded inconsistent results about the adverse perinatal outcomes of prematurity, stillbirths, and early pregnancy losses (8-11). A study on 1140 pregnant women in India showed that 88% of pregnant women positive for SARS-CoV-2 were asymptomatic. The researchers concluded that international guidelines are needed for testing (12). Furthermore, a systematic review indicated that maternal mortality rose by 37% during the pandemic compared to before (13). However, only two studies from India reported a significant increase in maternal deaths (14). One study demonstrated a 7% rise in maternal mortality, while the results of another study did not show any significant rise during the pandemic (15).
Neonatal SARS-CoV-2 infections are sporadic, and the possibility of the vertical transmission of infection from mother to infant is not clear (16, 17). However, studies have found contradictory results on the vertical and/or postnatal transmission of SARS-CoV-2 (18, 19). There is also no conclusive evidence of the transmission of SARS-CoV-2 through the placenta and breast milk (6, 20). Other investigations have shown the presence of SARS-CoV-2 by polymerase chain reaction (PCR) in the upper respiratory tract of neonates with mild respiratory disease (21, 22). Studies assessing the impact of COVID-19 on maternal characteristics, pregnancy outcomes, and vertical transmission were inconsistent.
2. Objectives
The present study was designed to understand the clinical characteristics and outcomes of neonates born to mothers positive for SARS-CoV-2.
3. Methods
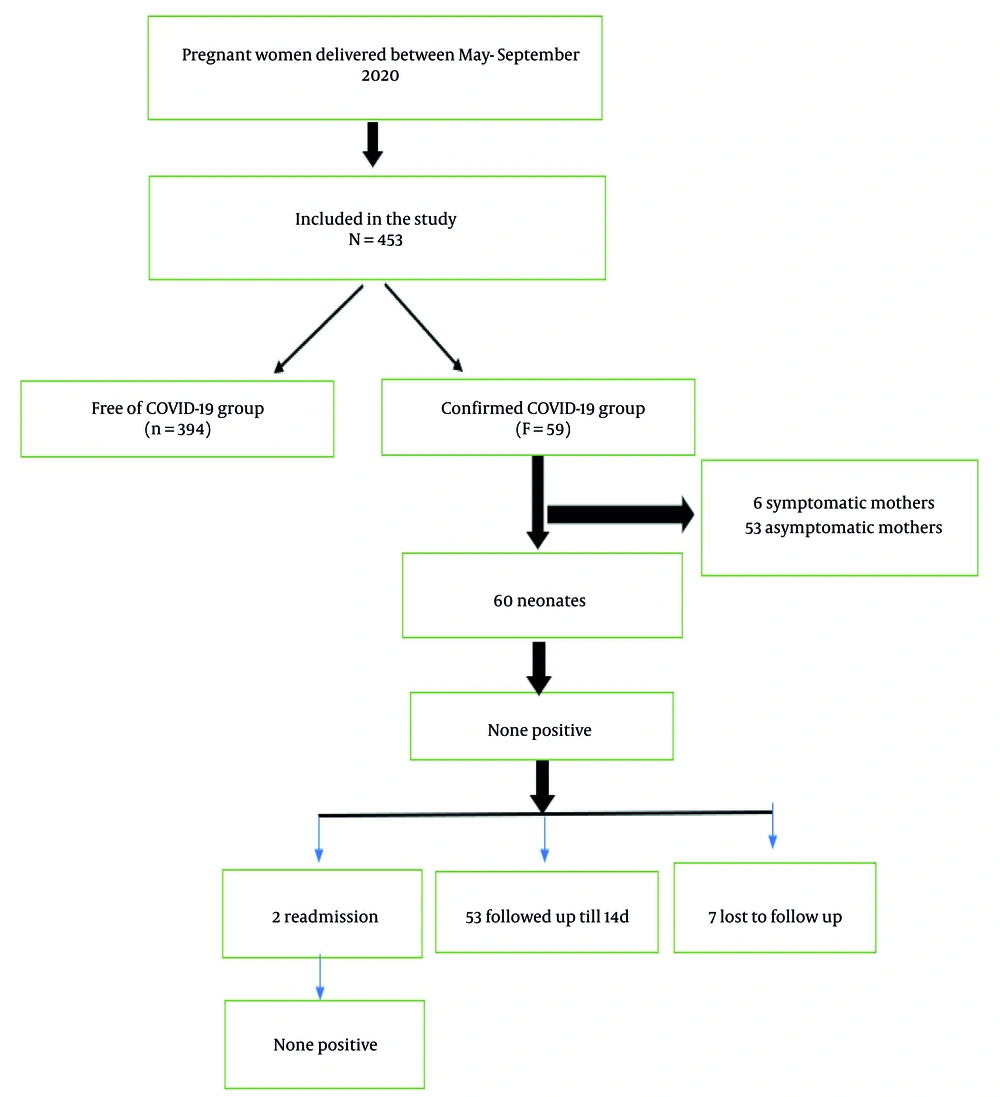
This study was conducted during May 1, 2020-September 30, 2020, at Ramaiah Medical College Hospital, Bangalore, Karnataka, India. The flowchart for the recruitment of subjects is presented in Figure 1. All pregnant women delivered at this tertiary care hospital during the study period were included. Informed consent was obtained from all patients prior to the study. The ethical confirmation for the study was taken from the Institutional Ethics Committee (IEC: MSRMC/EC/AP-01/12-2020). Nasopharyngeal swabs from all pregnant women were tested for SARS-CoV-2 using reverse transcriptase (RT)-PCR (NeoDx, Biotech labs kit, India) assay at admission to Labor and Delivery.
All pregnant mothers who were positive for SARS-CoV-2 or were suspected of having SARS-CoV-2 infection were referred to an isolated labor room or isolated operating theatre. The maternal information, including age, preterm premature rupture of membranes (PPROM), gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH)/preeclampsia, and antepartum hemorrhage (APH), were obtained from medical records or direct communication with patients and their families. All pregnant women positive for SARS-CoV-2 were divided into two groups, including symptomatic and asymptomatic positive cases. Furthermore, laboratory findings for mothers, such as cough, PPROM, GDM, PIH/PE, and APH, were evaluated. In addition, some factors for neonates, including birth weight, mode of delivery, CPAP, MV, surfactants, WBC count, platelet count, and C-reactive protein (CRP), were collected.
The neonates born to positive or suspected mothers were shifted to an isolation nursery or neonatal intensive care unit (NICU) based on the clinical condition of the baby. A neonatal throat swab was collected to test for SARS-CoV-2 by RT-PCR at 24 - 48 hours of life. Repeat swab testing was completed only if the baby developed symptoms within 2 weeks of delivery. Neonates were started on expressed breast milk from the mother or formula feeds. Infants having any clinical symptoms were managed based on the standard NICU protocol. If the neonate was asymptomatic and tested negative, discharge was planned along with the mother with the advice to follow up for any symptoms at 2 weeks of life. The follow-up information was obtained by telephone and video consultation with the neonates. If the neonate was asymptomatic and negative for SARS-CoV-2 and the mother was not discharged from the hospital, the option of the baby being discharged with a healthy caretaker (not a primary contact) was discussed with the parents.
3.1. Statistical Analysis
All data were analyzed using the SPSS software version 16 (SPSS Inc., Chicago, IL, USA). Descriptive data are presented as number and percentage, mean and standard deviation, or median and inter-quartile range. The chi-square test was used for categorical variables, an independent t-test was utilized for variables with normal distribution, and the Mann-Whitney test was applied for variables with non-normal distribution. P-value < 0.05 was considered statistically significant.
4. Results
During the study period, 453 pregnant women were admitted to the hospital mentioned above for delivery and were evaluated by the obstetric team. Among them, 59 (13.1%) women were confirmed with SARS-CoV-2 and underwent diagnostic evaluation, and the remaining 394 (86.9%) were negative for the virus. Table 1 shows the study characteristics comparison between the positive and negative pregnant women. There was a statistically significant difference for PPROM (P = 0.001) and GDM (P = 0.006) between the pregnant women positive and negative for SARS-CoV-2. However, no significant difference was observed for age, PIH, and APH (Table 1).
| Characteristics | SARS-CoV-2 | P-Value | |
|---|---|---|---|
| Negative (N = 394) | Positive (N = 59) | ||
| Age (y) | 28.6 ± 4.54 | 29.1 ± 4.9 | 0.524 |
| PPROM | 0.001 | ||
| Yes | 25 (6.3) | 12 (20.3) | |
| No | 369 (93.7) | 47 (79.6) | |
| GDM | 0.006 | ||
| Yes | 46 (11.7) | 15 (25.4) | |
| No | 348 (88.3) | 44 (74.5) | |
| PIH/PE | 1 | ||
| Yes | 71 (18) | 11 (18.6) | |
| No | 323 (82) | 48 (81.3) | |
| APH | 1 | ||
| Yes | 5 (1.3) | 1 (1.69) | |
| No | 389 (98.7) | 58 (98.3) | |
Abbreviations: PPROM, preterm premature rupture of membranes; GDM, gestational diabetes mellitus; PIH, pregnancy-induced hypertension; PE, preeclampsia; APH, antepartum hemorrhage.
a Values are expressed as No. (%) or mean ± SD.
Moreover, the characteristics of neonates stratified by maternal SARS-CoV-2 status are presented in Table 2. The mode of delivery was the only characteristic that showed a statistically significant difference between pregnant women with or without SARS-CoV-2 (P = 0.002) (Table 2). In addition, among the positive pregnant women, 74.6% of neonates were born by C-section. The other characteristics, such as birth weight, need for NICU admission, need for respiratory support, and the duration of hospital stay, were not found to have significant differences between the study groups (Table 2).
| Characteristics | SARS-CoV-2 | P-Value | |
|---|---|---|---|
| Negative (N = 402) | Positive (N = 59) | ||
| Gestational age (w) | 28.9 ± 4.3 | 29.1 ± 4.9 | 0.525 |
| Birth weight (kg) | 2.79 ± 0.64 | 2.9 ± 0.64 | 0.512 |
| Respiratory support | 0.37 | ||
| Yes | 72 (17.9) | 14 (23.7) | |
| No | 330 (82.1) | 45 (76.2) | |
| Hospital stay (d) | 0.481 | ||
| Yes | 5.42 ± 6.65 | 5.95 ± 5.21 | |
| NICU | 0.604 | ||
| Yes | 81 (20.1) | 14 (23.7) | |
| No | 321 (79.8) | 45 (76.2) | |
| Mode of delivery | 0.002 | ||
| LSCS | 198 (49.3) | 44 (74.6) | |
| NVD | 204 (50.7) | 15 (25.4) | |
| O2 by hood | 0.451 | ||
| Yes | 64 (15.9) | 12 (20.3) | |
| No | 338 (84.1) | 47 (79.7) | |
| CPAP | 0.406 | ||
| Yes | 27 (6.7) | 2 (3.4) | |
| No | 375 (93.3) | 57 (96.6) | |
| MV | 0.378 | ||
| Yes | 9 (2.2) | 3 (5.1) | |
| No | 393 (97.8) | 56 (94.9) | |
| Surfactant | 1 | ||
| Yes | 12 (3) | 2 (3.4) | |
| No | 390 (97) | 57 (96.6) | |
| APGAR score | 8.39 ± 0.79 | 8 ± 1.23 | 0.029 |
Abbreviations: NICU, neonatal intensive care unit; CPAP, continuous positive airway pressure; MV, mechanical ventilation; APGAR, appearance, pulse, grimace, activity, and respiration.
a Values are expressed as No. (%) or mean ± SD.
A total of 59 pregnant women with SARS-CoV-2 were classified into two groups based on being symptomatic (10.1%) or asymptomatic (89.8%), and their clinical characteristics are summarized in Table 3. Cough (P = 0.001) and PPROM (P = 0.024) had significant associations with SARS-CoV-2 infection in pregnant women. Furthermore, age (P = 0.655) and GDM (P = 0.999) did not show any significant association with the groups (Table 3). Table 4 demonstrates 60 (1 mother delivered twin babies) characteristics of neonates stratified by maternal symptomatic and asymptomatic SARS-CoV-2. There were no significant differences in the studied characteristics between the symptomatic and asymptomatic cases (Table 4).
| Characteristics | SARS CoV-2 Positive | P-Value | |
|---|---|---|---|
| Symptomatic (N = 6) | Asymptomatic (N = 53) | ||
| Age (y) | 21.8 ± 4.8 | 29.1 ± 4.9 | 0.655 |
| Cough | 0.001 | ||
| Yes | 5 | 0 | |
| No | 1 | 53 | |
| PPROM | 0.024 | ||
| Yes | 4 | 10 | |
| No | 2 | 43 | |
| GDM | 0.999 | ||
| Yes | 2 | 13 | |
| No | 4 | 40 | |
| PIH/PE | 0.344 | ||
| Yes | 0 | 11 | |
| No | 6 | 42 | |
| APH | 1 | ||
| Yes | 0 | 1 | |
| No | 6 | 52 | |
Abbreviations: PPROM, preterm premature rupture of membranes; GDM, gestational diabetes mellitus; PIH, pregnancy-induced hypertension; PE, preeclampsia; APH, antepartum hemorrhage.
| Characteristics | SARS-CoV-2 Positive | P-Value | |
|---|---|---|---|
| Symptomatic (N = 6) | Asymptomatic (N = 53) | ||
| Gestational age (w) | 28.2 ± 4.9 | 27.8 ± 3.1 | 0.656 |
| Birth weight (kg) | 2.7 ± 0.54 | 2.9 ± 0.6 | 0.446 |
| Mode of delivery | 1 | ||
| LSCS | 5 | 39 | |
| NVD | 1 | 14 | |
| O2 by hood | 0.091 | ||
| Yes | 3 | 9 | |
| No | 3 | 44 | |
| CPAP | 1 | ||
| Yes | 0 | 2 | |
| No | 6 | 51 | |
| MV | 1 | ||
| Yes | 0 | 3 | |
| No | 6 | 50 | |
| Surfactants | 1 | ||
| Yes | 0 | 2 | |
| No | 6 | 51 | |
| CRP | 0.67 | ||
| Positive | 5 | 38 | |
| Negative | 1 | 15 | |
| Discharge weight | 2.6 ± 0.52 | 2.8 ± 0.5 | 0.284 |
| WBC | 14616 ± 5998 | 22266 ± 28976 | 0.109 |
| Platelet | 246666 ± 6742 | 242076 ± 76509 | 0.881 |
| APGAR score | 8 ± 0.7 | 8.15 ± 0.8 | 0.659 |
Abbreviations: CPAP, continuous positive airway pressure; MV, mechanical ventilation; CRP, C-reactive protein; WBC, white blood cells; APGAR, appearance, pulse, grimace, activity, and respiration.
We reached 52 parents out of 59 confirmed maternal SARS-CoV-2 cases (88%) through phone calls and video consultation by the neonatal team on day 14 of life to determine the neonate's clinical condition and feeding status. Out of 53 neonates (1 pair of twins included) born to mothers positive for SARS-CoV-2, 7 (13%) were on formula feeds, and the rest (87%) were on exclusive breastfeeding. Two neonates were readmitted for exaggerated physiological jaundice needing phototherapy. Both neonates tested negative for SARS-CoV-2 when tested again on their readmission to the NICU.
5. Discussion
We compared the pregnancy outcomes between the pregnant women positive and negative for SARS-CoV-2. In addition, clinical characteristics were compared between the symptomatic and asymptomatic pregnant women infected by SARS-CoV-2. In the present study, an increase was observed in the percentage of C-section delivery among pregnant women with SARS-CoV-2. However, there were no neonates infected by SARS-CoV-2 in either of the study groups. Cough was the only symptom observed in the infected pregnant women. The PPROM was significantly different between positive and negative women and between symptomatic and asymptomatic groups.
Previous studies reported unique physiologic and immunologic changes and augmented risk for several viral infections, including influenza and SARS during pregnancy (23, 24). Furthermore, a study that compared pregnant and non-pregnant women revealed that pregnant women were more vulnerable to infection and developed grievous disease complications (25). In the current study, the SARS-CoV-2 infection rate among pregnant women was 13%. Another study in Maharashtra, India, on 1140 pregnant women reported 141 participants as positive for SARS-CoV-2, resulting in a prevalence of 12.3%. Variable prevalence rates (0 - 40%) were reported across different hospitals within the state (12). Although several hospital-based investigations showed that SARS infection increased the mortality rate in pregnant women (26), no deaths were reported among 59 confirmed SARS-CoV-2 cases in the current study. However, another study conducted in Hong Kong, China, found that SARS-CoV-2 infection in pregnant women caused higher pregnancy loss rates, prematurity, growth restriction in the fetus, and a 25% fatality rate (23).
The prevalence rate of symptomatic pregnant women was 10.1%, while that of asymptomatic pregnant women was 89.8% in the current study population. The results are in accordance with the previous study conducted in Maharashtra, India, which found the prevalence of symptomatic and asymptomatic pregnant women to be 11.5% and 88.5%, respectively (12). The prevalence of symptomatic and asymptomatic SARS-CoV-2 pregnant women greatly varied across distinct cities. A study on 1148 women hospitalized for SARS-CoV-2 during pregnancy showed that 63% were symptomatic. The incidence of hospital admissions for symptomatic SARS-CoV-2 infection was 2 per 1000 maternity and for asymptomatic was 1.2 per 1000 maternity (27).
A study on a large population of 2143 patients showed that over 90% were either asymptomatic or had mild/moderate disease. However, 10.6% of severe cases were reported in the age group under 1 year, indicating that the risk of infants developing severe respiratory failure may be higher among children (28). So far, there has been no proper conclusion on the intrauterine and breastfeeding transmission of SARS-CoV-2 from mother to infant. Chen et al. tested six SARS-CoV-2-infected neonatal samples of cord blood, amniotic fluid, and throat swabs. These authors showed no evidence of transmission from mother to fetus (18). Furthermore, Liu et al. (29) reported no serological evidence for the mother-to-fetus transmission of the virus. Zhu et al. (30) revealed PCR-negative throat swabs for SARS-CoV-2 in ten neonates. On the other hand, a case report based on positive IgM serology in a neonate suggested intrauterine infection.
In our study, maternal comorbidities, such as PPROM, GDM, PIH, and APH, were compared between the groups negative and positive for SARS-CoV-2. We observed a significant difference in GDM between the groups negative and positive for SARS-CoV-2. However, no difference was found between the symptomatic and asymptomatic groups. The Centers for Disease Control reported more diabetes complications in pregnancy among women hospitalized for SARS-CoV-2 infection than those hospitalized for other obstetric reasons (8.1%) (31). A retrospective observational study found 14.3% PIH or preeclampsia cases, 3.6% pregestational diabetes mellitus, and 10.7% GDM among individuals positive for SARS-CoV-2. However, none of these risk factors were significantly different between the pregnant women positive and negative for SARS-CoV-2 (32, 33).
Data is limited concerning the relationship between the mode of delivery and perinatal transmission of COVID-19. Three neonates were born vaginally, and throat swabs on day one of birth were negative for SARS-CoV-2 by PCR (29). Another patient positive for SARS-CoV-2 was negative for the virus tested by vaginal swab during delivery (34). Therefore, the available data suggest no increase in the risk of perinatal vaginal birth to neonates. Several reports on postpartum transmission confirm that neonates can become infected with SARS-CoV-2 through horizontal transmission via respiratory droplets or close contact with parents or caregivers (25, 35). Moreover, the breast milk samples of mothers infected by SARS-CoV-2 had negative PCR results (18). In the current study, no SARS-CoV-2-positive infants were observed, which may be due to strict infection control and prevention procedures implemented during delivery. For example, the immediate separation of infants from infected mothers could be a possible explanation. In our study, the measures to avoid infection transmission from infected mothers to neonates might have reduced the exclusive breastfeeding rates at discharge and follow-up. However, the post-discharge advice given to the mothers effectively reduced the rate of postnatal horizontal transmission of the virus, resulting in none of the neonates being symptomatic for SARS-CoV-2 and reduced readmission rates.
This study had some limitations, the first of which was the very small sample size and the low number of SARS-CoV-2 patients. Second, this was a single-center study necessitating the assessment of different populations in the same geographic region. Finally, proper follow-up for the negative patients should be taken into consideration because this is a pandemic disease, and the chance of infection after discharge is high.
5.1. Conclusions
The present study provided evidence for pregnant women infected by SARS-CoV-2 and their neonate’s clinical outcomes. There were no neonates infected with SARS-CoV-2 from infected pregnant women. The number of asymptomatic pregnant women was higher than symptomatic cases in the current research. Moreover, an increased percentage of C-section delivery was found among the pregnant women infected by SARS-CoV-2. However, the long-term follow-up of pregnant women and their children and multicenter investigations, which include a large number of samples in different centers across the country, are required to confirm our results. Furthermore, extensive research to evaluate the long-term outcomes and potential of the vertical transmission of SARS-CoV-2 to infants are warranted.