1. Background
Rattus norvegicus (R. norvegicus) are recognized to be reservoirs for different zoonotic diseases and are associated with important hygienic problems (1). In urban environments, R. norvegicus often live in proximity to human populations and are linked to significant human morbidity and mortality in developing and developed countries (2). These rodents carry different microorganisms including bacteria, viruses, and parasites and they are considered to be a concern for global public health in urban environments (3). So far, 79 different species of rodents have been identified in Iran (2). Among these rodents, R. norvegicus had the highest frequency and were frequently isolated in urban areas. It has been revealed that 61% of 1500 known human pathogens are common among humans and animals. Therefore, these pathogens can cause zoonotic diseases (4). In general, the rodent’s population contaminates water and food and can infect the human population in different ways. However, contact with different rat secretions such as urine, saliva, and feces, consumption of contaminated water or food, inhalation of aerosols, direct contact by bites, direct contact with contaminated infected domestic animals, and infections via vectors are considered the main pathways (5-7). Based on several studies conducted in different countries, R. norvegicus represent a reservoir for Leptospira spp. Moreover, it is predicted that these rodents carry other microorganisms such as Vibrio vulnificus (V. vulnificus) and Rabies virus (3, 8, 9). Despite the existence of an effective vaccine regimen, the rabies virus continues to be a global health concern with an estimated human death rate of 55,000 each year, worldwide (10). V. vulnificus can cause severe infections from gastroenteritis to ‘primary sepsis’ and necrotizing fasciitis and is associated with most human death cases caused by Vibrios (11). Leptospirosis is an important endemic zoonotic disease, has a global distribution, and is considered a public health concern (12). Based on the World Health Organization’s (WHO’s) Leptospirosis Burden Epidemiology Reference Group reports, approximately 60,000 human deaths occur each year, worldwide (13). Direct contact with rodent population or infected livestock and wild animals, contact with surface water, soil, and plants, consumption of contaminated water, direct contact with the urine of infected animals, or contact with a urine-contaminated environment are the main ways that Leptospira spp. can infect children (14). It has been found that due to frequent exposure to surface water and contact with animals, children represent a more susceptible group to Leptospira infections (13). Tehran, home to a population of 10 - 12 million people, is the most populous city in Iran and Western Asia that has a hot-summer Mediterranean climate (1). However, data about the prevalence of Leptospira spp., V. vulnificus, and Rabies virus in urban rat populations are limited and remain unexplored. The current study carried out a pilot survey of rats collected from five districts of Tehran for Leptospira spp., V. vulnificus, and Rabies virus.
2. Objectives
The present study aims to determine the presence and frequency of these agents in the R. norvegicus population living in an urban environment.
3. Methods
3.1. Ethics Approval
The present study was approved by the Ethics Committee of the National Institutes for Medical Research Development (NIMAD) with reference number IR.NIMAD.REC. 1396.323.
3.2. Study Sites and Sample Collection
During one year from May 2018 to May 2019, 100 R. norvegicus were captured from five districts of Tehran, Iran, including 20 from the northern district, 20 from the southern district, 20 from the western district, 20 from the eastern district, and 20 from the central district. All the trapping locations were selected in urban areas in alleys behind residential dwellings. Within each trapping location, Sherman live traps and alluring baits were set and sampling was done through a convenient sampling method. The sampling strategy will be designed to trap similar numbers of rats in five districts. The sampling will be performed after sundown in every five districts and processed during the next morning. All rats were transported to a guaranteed special laboratory in animal houses within 48 h after being captured and fecal samples were collected and transferred to top-screwed small bottles containing formol-ether solution and labeled. All rats were euthanized by the intramuscular injection of ketamine and xylazine (0.1 mg/kg) followed by bilateral thoracotomy. In the next step, blood samples were collected by cardiac puncture using a 5ml syringe, and serum was recovered after centrifugation. All fecal and serum samples were stored at -80°C until molecular and serological analysis.
3.3. Enzyme-linked Immunosorbent Assay
Briefly, all serum samples were screened for specific antibodies against Leptospira spp. and Rabies virus by Enzyme-Linked Immunosorbent assay (ELISA). The ELISA method was performed using a commercial qualitative rat ELISA kit (Shanghai Crystal Day Biotech Co., Ltd) at the central laboratories of the Department of Microbiology, Shahid Beheshti University of Medical Sciences. The optical density (OD value) of each well was read with a spectrophotometer at 450/620 nm within 15 min of stopping (15 min after adding the sulfuric acid). All of the ELISA steps and the process of determining the cut-off amount were performed according to the manufacturer’s instructions.
3.4. DNA Extraction and Polymerase Chain Reaction
Genomic DNA was extracted from fecal samples using the DNA extraction kit (AllPrep DNA minikit (Qiagen, Inc.), as recommended by the manufacturer. All extracted DNA samples were eluted in 50 µl of elution buffer and stored at -80°C before Polymerase chain reaction (PCR) analysis. In the next step, PCR was performed to determine the prevalence of V. vulnificus using specific primer pairs including F: 5’-TTCCAACTTCAAACCGAACTATGA-3’ and R: 5’-ATTCCAGTCGATGCGAATACGTTG -3. Briefly, 25 µL PCR mixture consists of 12 µL of 2× Master Mix (Amplicon, Pishgam, Tehran, Iran; Cat. no. PR901638) including 0.5 µL of 10 mM of each deoxynucleoside triphosphate (dNTPs), 1 × PCR buffer, 3 mmol/L MgCl2, 1 unit of Taq DNA polymerase, 0.5 µM of each primer (10 mM), 3 µL of template DNA, and 9 µL of sterile distilled water. PCR reactions were performed on a thermal cycler (Eppendorf, Mastercycler Gradient; Eppendorf, Hamburg, Germany). PCR was conducted under the following condition: one cycle at 95°C for 5 minutes, 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 45 s, 72°C for 45 s, and the final extension step at 72°C for 10 minutes following the last cycle. All PCR products were electrophoresed on a 1.5% agarose gel, visualized by DNA safe stain (SinaClon Co., Iran), and photographed under UV light (Life Technologies). The positive PCR products representing the studied gene were confirmed by sequencing analysis (Macrogen Korea). The results of sequencing were studied by the NCBI BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/).
3.5. Statistical Analysis
All data were included in an SPSS file, and the prevalence of surveyed microorganisms was analyzed by the statistical package SPSS V.23.0 (SPSS Inc., Chicago, IL, USA) using descriptive statistic tests.
4. Results
4.1. Prevalence of Zoonotic Pathogen
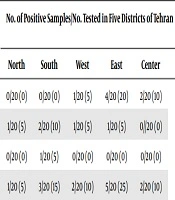
During the one year from May 2018 to May 2019, 100 R. norvegicus were captured from five districts (North, South, West, East, and center) of Tehran, Iran. In general, 80% (n = 80/100) and 20% (n = 20/100) of rats were males and females, respectively. The prevalence and distribution of surveyed microorganisms among R. norvegicus in five districts of Tehran are shown in Table 1.
| Zoonotic Parasites | Sample Type | Methods | No. of Positive Samples / No. of Tested in Five Districts of Tehran | |||||
|---|---|---|---|---|---|---|---|---|
| North | South | West | East | Center | Total | |||
| Leptospira spp. | Serum | ELISA | 0/20 (0) | 0/20 (0) | 1/20 (5) | 4/20 (20) | 2/20 (10) | 7/100 (7) |
| V. vulnificus | Fecal | PCR | 1/20 (5) | 2/20 (10) | 1/20 (5) | 1/20 (5) | 0//20 (0) | 5/100 (5) |
| Rabies virus | Serum | ELISA | 0/20 (0) | 1/20 (5) | 0/20 (0) | 0/20 (0) | 0/20 (0) | 1/100 (1) |
| Total | - | - | 1/20 (5) | 3/20 (15) | 2/20 (10) | 5/20 (25) | 2/20 (10) | 13/100 (13) |
Numbers of Rattus norvegicus and Sample Types Positive for Surveyed Zoonotic Microorganisms Identified by ELISA and PCR Methods in five Districts of Tehran
Moreover, the frequency of surveyed pathogens among male and female R. norvegicus is shown in Table 2. Results showed that 13% (n = 13/100) of all captured rats were positive from which different surveyed microorganisms were isolated. The presence of specific rat IgG antibodies against Leptospira spp. and Rabies virus was detected using an ELISA kit. In total, results of the ELSA assay showed that of the 100 R. norvegicus captured in Tehran, 7% (n = 7/100) and 1% (n = 1/100) were positive for Leptospira spp. and Rabies virus, respectively. Among the five districts, the frequency of Leptospira spp. is as follows: North (0%; 0/20), south (0%; 0/20), west (5%; 1/20), east (20%; 4/20), and center (10%; 2/20). Leptospira spp. revealed the highest frequency among R. norvegicus collected from the eastern part of Tehran. Rabies virus was only detected from the Southern (5%; 1/20) part of Tehran. PCR assay was applied to survey the presence of V. vulnificus in fecal samples collected from R. norvegicus. Results revealed that the percentage of the rats tested positive for V. vulnificus in the five districts of Tehran was 5%. The frequency of V. vulnificus among five districts was as follows: North (5%; 1/20), south (10%; 2/20), west (5%; 1/20), and east (5%; 1/20). V. vulnificus was not detected in the central parts of Tehran. Overall, the surveyed zoonotic microorganisms had the highest (n = 5/20; 25%) and lowest (n = 1/20; 5%) frequency rates among R. norvegicus collected from the eastern and northern parts of Tehran, respectively.
| Parasites | Total Positive, % | Positive Cases Among Genders, % | |
|---|---|---|---|
| Male | Female | ||
| Leptospira spp. | 7 | 100 | 0 |
| V. vulnificus | 5 | 100 | 0 |
| Rabies virus | 1 | 100 | 0 |
The Frequency of Surveyed Microorganisms Among Male and Female R. norvegicus
5. Discussion
In urban areas, rodents exist in large populations and live with and near human populations (2). R. norvegicus is recognized as the reservoir for different human zoonotic pathogens and has a significant role in zoonotic disease ecology, worldwide (1). In the current study, R. norvegicus were captured from five districts in Tehran to obtain data about the presence and frequency of Leptospira spp. V. vulnificus and Rabies virus. The results showed that Leptospira spp. were the main zoonotic pathogens that were (7%; n = 7/100) isolated from the R. norvegicus population in Tehran. Besides, the highest prevalence (20%; n = 4/20) of Leptospira spp. was identified in the eastern part of Tehran. Several studies have surveyed the presence and prevalence of Leptospira spp. in Rattus in cities around the world. Azhari et al. (15) surveyed the prevalence and molecular characterization of pathogenic Leptospira spp. in the Rattus population captured from Selangor state, Malaysia. The finding of their study revealed an overall Leptospira detection rate of 14.3% among the 266 Rattus captured. Results of another study conducted by Firth et al. (3) in the USA revealed that the prevalence of Leptospira interrogans among R. norvegicus was 12%. Maas et al. (8) in 2018 reported that the prevalence of Leptospira spp. in R. norvegicus in four regions in the Netherlands was 33% - 57%. Costa et al. (16) in Brazil and Runge et al. (17) in Germany found that the frequency of Leptospira spp. in Norway rats was 83% and 25%, respectively. In Iran, Mosallanejad et al. (18) surveyed the frequency of Leptospira infection among Rattus rattuss of Ahvaz District, southwest of Iran. The results of their study revealed that from a total of 120 Rattus rattuss, 3.3% were serologically positive to Leptospira infection. In general, rodent population and domestic animals such as pigs, dogs, and cattle are considered as the reservoirs for and carriers of Leptospira spp. (12). Leptospira spp. can infect humans in several ways including direct contact with urine or feces of infected animals as well as contact with contaminated soil and water (19). Children spend more time playing games in contaminated surface water and animal. They are considered as a group susceptible to leptospirosis and run an increased risk of contracting this zoonotic disease (13, 14, 20, 21). Leptospira spp. can cause febrile illnesses and lead to high mortality and morbidity in children. It has been revealed that fever, myalgia, gastroenteritis-like symptoms, and conjunctival suffusion are common clinical features of leptospirosis among children (22). The difference in the prevalence rate of Leptospira spp. among conducted studies results from several factors such as (1) variation in samples type; (2) difference in diagnostic methods; (3) different sanitary conditions of the urban environment in countries; (4) awareness levels of peoples about the rat-borne disease; (5) climate variations and different rainfall patterns among countries; and (6) education status and poverty (22, 23).
In the present study, V. vulnificus had the highest frequency (10%; n = 2/20) in the R. norvegicus population captured from the southern part of Tehran. The total frequency of V. vulnificus was 5% (n = 5/100). To the best of our knowledge, the current study is the first research to have reported the prevalence of V. vulnificus in the R. norvegicus population, worldwide. In 2014, Firth et al. in the USA investigated the frequency of V. vulnificus in Norway rats. However, the results of their research revealed that none of the samples tested was positive for V. vulnificus (3). V. vulnificus can cause a potentially fatal disease among children, especially among those with a weakened immune system. Therefore, rapid diagnosis, immediate management, and better treatment of disease are critical (11, 24, 25). The frequency of the Rabies virus among the R. norvegicus population was 1%. In general, we found two different studies conducted by Kantakamalakul et al. (26) in Thailand and Patabendige and Wimalaratne (27) in Colombo, who surveyed the prevalence of Rabies virus among local rodents and domestic rats. Results of both studies revealed that the rabies antigen was not detected in any rodent population.
The results of the present study revealed that the Rattus population carried the zoonotic organisms in the urban environment. Children are vulnerable to infectious diseases and can be infected with different zoonotic pathogens in several ways. The limitation of this study lies in the small number of rodents in each geographical region in Tehran. This limitation is associated with the difficulty of capturing such rodents.
5.1. Conclusions
The finding of the current study emphasizes a number of important steps: (1) implementation of better rodent control programs in urban environments; (2) the need to prevent children from playing outside and in contaminated soil or water; (3) disinfection of urban areas; (4) the necessity of avoiding contact with Rattus and other rodent population in urban environments for children, especially immunocompromised patients; and (5) suitable maintenance of sanitary conditions and adoption of better waste disposal measures. Since this study is a pilot survey, the results of the present study point to the need for conducting further studies on a larger scale in Tehran, Iran.