1. Background
IL-17 producing T cells, initially identified in 2005 (1-3), are a CD4+ T cell lineage characterized by the production of IL-17A (4). IL-17 producing cells not only secrete IL-17A and IL-17F but also produce IL-21, IL-22, IFN-γ, IL-4, IL-10, IL-9, IL-26, CXCL8, and CCL20 (4). In humans, the reputation of IL-17 producing T cells was due to their increase at the sites of inflammation (5). While IL-17 producing T cells were initially considered a destructive element in many disorders such as psoriasis and multiple sclerosis (6), some data have suggested them as a unique effector T cell subset critical in protective immunity against fungi (eg, Candida albicans) and extracellular bacteria (eg, S. aureus) (7, 8). Specific defects in the IL-17 pathway have been described, each representative of a clinical phenotype attributable to a primary immunodeficiency syndrome. In hyper-IgE syndrome caused by mutations in STAT3, the underlying cellular basis for the characteristic phenotype is the lack of IL-17 producing T cells in the peripheral blood (9) due to low expression levels of RORC2, which is essential for the differentiation of naïve TH cells into IL-17 producing T cells. In chronic mucocutaneous candidiasis, chronic or persistent infections with C. albicans and S. aureus were found to occur due to IL-17RA autosomal recessive deficiency or IL-17F autosomal dominant deficiency and low numbers of peripheral IL-17 producing T cells (10). Low numbers of IL-17 producing T cells have also been detected in patients with CARD9 deficiency, which is essential for the signaling of dectin-1 (11).
2. Objectives
In this study, we sought the number of IL-17 producing T cells in patients with candida infections suspected to have an underlying primary immunodeficiency to evaluate any decrements in the number of IL-17 producing T cells as a predictor of an underlying primary immunodeficiency and as an indication for further complicated investigations including genetic studies.
3. Methods
Seven newly diagnosed patients with documented candida infections (two males and five females) were included in this study, with their ages ranging from 4 to 35 years (mean = 15.71). All patients with fungal infections were referred to the immunodeficiency clinic of Mofid Children's Hospital for further evaluation from 2014 to 2015. Recurrent fungal infections were detected in the course of the disease of these patients and were finally confirmed by fungal culture. A thorough laboratory workup consisting of immunodeficiency screening tests including complete blood count, serum immunoglobulin levels and antibody responses, flow-cytometry evaluation, and Lymphocyte Transformation Test were completed for all the patients, which accordingly, combined immunodeficiencies and neutrophil defects were excluded. Meanwhile, seven age- and sex-matched healthy volunteers were asked to participate in the study as the control group. After taking informed consent from all the participants, the percentage of IL-17 producing T cells was detected in the peripheral blood of all the patients and the healthy controls.
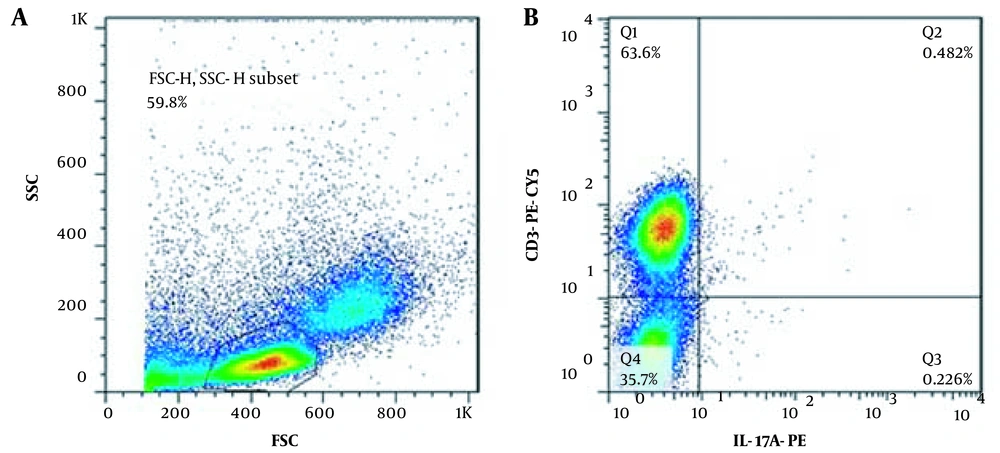
The patients' information, including demographic data, parental consanguinity, clinical manifestations, the time and site of the initiating symptoms, the duration of the fungal infection, and the natural course of the disease, were recorded by a single researcher with personal clinical experience in this field to confirm the eligibility of the patients to participate in the study. Fresh venous blood was drawn in 10 ml EDTA tubes from the patients, and the healthy controls Peripheral Blood Mononuclear cells were isolated from the patients’ venous blood via Ficoll 1.077 (Biowest, Austria) gradient centrifugation. A total number of 6 × 106 PBMCs were stimulated with ionomycin (10-5 molar) and PMA (40 ng/ML) (both from Sigma-Aldrich, US) at 37°C with 5% CO2 for 12 hours. One hour after stimulation with ionomycin and PMA, Golgiplug (BD biosciences, US) was added to prevent cytokine release. After incubation, the cells were surface stained with anti CD3-PE-CY5 (BD biosciences, US). Then, they were fixed and permeabilized using BD cytofix/cytoperm solutions (BD biosciences, US). Afterward, intracellular IL-17 was stained using an anti-Il-17A-PE antibody (BD Biosciences, US). IL-17 producing T cells were identified as CD3+IL-17A+ lymphocytes.
The patients’ data were analyzed using SPSS version 19. The Mann-Whitney test was used to detect differences between healthy controls and patients. A P-value of less than 0.05 was considered statistically significant.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the Institutional Ethics Committee of the Mofid’s Children Hospital.
4. Results
We analyzed the quantity of circulating IL-17 producing T cells in patients with Candida infections associated with underlying primary immunodeficiency. One of the patients had a genetic diagnosis for CARD9 deficiency, and another patient fulfilled the clinical criteria for Job's syndrome.
Tables 1 and 2 are summaries of clinical and laboratory data of the patients, respectively.
| No. | Age (y) | Gender | Consanguinity | Diagnosis | Signs & Symptoms |
|---|---|---|---|---|---|
| 1 | 18 | Male | + | CARD9 | Abdominal candidiasis |
| 2 | 35 | Female | - | CMCC | Candida vulvovginitis |
| 3 | 17 | Female | - | CMCC | Candida vulvovginitis |
| 4 | 4 | Male | - | CMCC | Nail candidiasis |
| 5 | 12 | Female | - | APECED | Recurrent fungal sinusitis and skin infections, Joint involvements, bronchiectasis, renal involvement, and adrenal insufficiencies |
| 6 | 14 | Female | + | CMCC | Nail candidiasis, nephritis |
| 7 | 10 | Female | + | HIES | Mouth & nail candidiasis, eczema, clubbing |
Abbreviations: CMCC, congenital mucocutaneous candidiasis; HIES, Hyper IgE syndrome; APECED, autoimmune polyendocrinopathies and ectodermal dysplasia; CARD9, caspase recruitment domain; NL, normal; ND, not determined).
| No. | IgGmg/dL | IgM mg/dL | IgAmg/dL | IgEmg/dL | Ab Response | LTT | CD3 (%) | CD4 (%) | CD16 (%) | CD 19 (%) | CD 16 (%) | WBC (Cells/ mcL) | ANC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1800 | 73 | 160 | 164 | NL | ND | ND | ND | ND | ND | ND | 4350 | 2200 |
| 2 | 1502 | 140 | 120 | 34 | NL | NL | ND | ND | ND | ND | ND | 5400 | 3300 |
| 3 | 996 | 80 | 78 | 26 | NL | ND | ND | ND | ND | ND | ND | 4800 | 2200 |
| 4 | 682 | 74 | 40 | 6 | NL | ↓Candida | ND | ND | ND | ND | ND | 6320 | 3400 |
| 5 | 1730 | 174 | 103 | 4 | ND | ND | 70 | 47 | 2.9 | 12.67 | 47 | 9800 | 3900 |
| 6 | 390 | 138 | 96 | 43 | ↓ | NL | 75.7 | 51 | 12.6 | 10 | 51 | 7240 | 3800 |
| 7 | 2019 | 73 | 220 | 8420 | NL | ↓Candida | 42.9 | 39.4 | 15.9 | 47.6 | 3.49 | 10700 | 5300 |
Abbreviations: NO, number; NL, normal; ND, not detected; LTT, lymphocyte transformation test; ↓, decreased; ANC, absolute neutrophil count.
Table 3 demonstrates the percentage of IL-17 producing T lymphocytes in patients with candidiasis and healthy controls.
| Number of Cases/Controls | Percentage of IL-17 Producing Lymphocytes in PBMCs of Immunodeficient Patients with Candidiasis | Percentage of IL-17 Producing Lymphocytes in PBMCs of Healthy Controls |
|---|---|---|
| 1 | 0.245 | 3.08 |
| 2 | 0.527 | 3.53 |
| 3 | 0.621 | 4.42 |
| 4 | 0.495 | 4.26 |
| 5 | 0.326 | 3.14 |
| 6 | 0.482 | 3.58 |
| 7 | 0.327 | 4.01 |
| Mean ± SD | 0.431857 ± 0.13444 | 3.707143 ± 0.504107 |
Abbreviation: PBMC, peripheral blood monocytes.
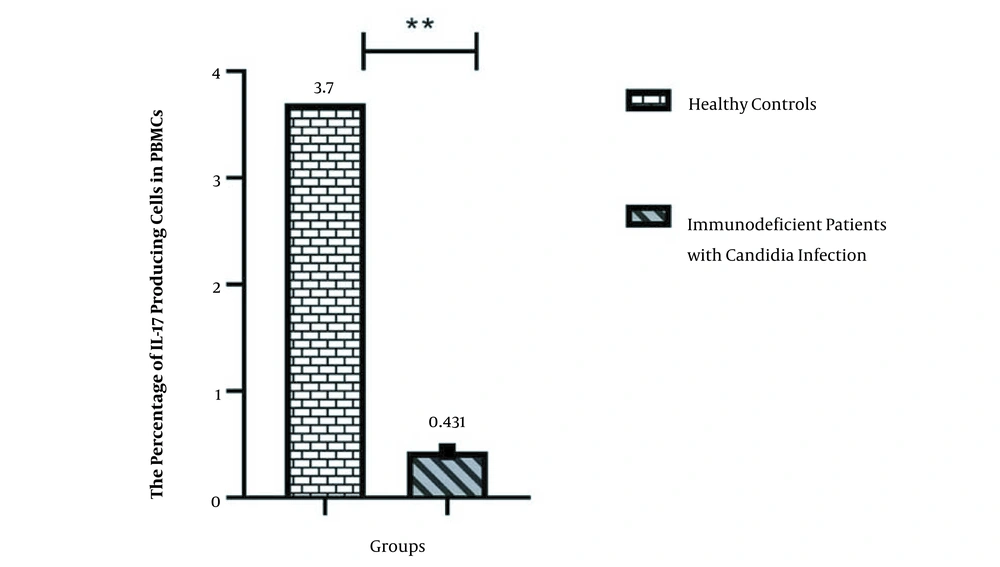
As shown, IL-17 producing T cells decreased in the peripheral blood of patients with primary immunodeficiencies associated with candida infections.
Figure 1 demonstrates a comparison of the percent of IL-17 producing T cells in normal healthy controls and immunodeficient patients with Candida infections.
Figure 2 represents the flowcytometric graph of one of the patients.
5. Discussion
This study aimed to evaluate the number of IL-17 producing T cells in patients with Candida infections and underlying primary immunodeficiency. A significant decrease in the number of IL-17 producing T cells compared to normal healthy controls was found in patients with specific Candida infections.
Recurrent mucocutaneous candidiasis has been known as a characteristic feature of several immunodeficiency syndromes, which implicate IL-17 producing T cells in immunity to candida, including acquired immunodeficiency syndrome (AIDS), hyper IgE syndrome (HIES), chronic mucocutaneous candidiasis (CMC), and CARD9 deficiency (12). Hernandez-Santos et al. found strong Candida-dependent recall responses generated in the CD4+ T-cell compartment. They showed that the development of IL-17 producing T cells in recall infections promoted accelerated fungal clearance (13). In another study, Delsing et al. showed that a deficiency in fungal induced IL-17 producing T cells’ responses caused Aspergillus osteomyelitis and attributed this event to the role of IL-17 producing T cells, which are crucial for neutrophil recruitment and controlling fungal invasion at the level of mucosae and skin (14, 15). These cells are part of the adaptive immune system, regulating innate immune responses. Therefore, it appears that in humans, IL-17 producing T cells have an essential role in protection against C. albicans since decreased IL-17 producing T lymphocyte population increases the susceptibility to candida infections (16, 17). However, our findings indicate a substantial decrease of IL-17 producing T cells in candida infections of patients with particular primary immunodeficiencies. A well-balanced response to C. albicans is initiated by IL-17 producing T cells dominating innate and adaptive mechanisms. Therefore, it is reasonable for extensive mucocutaneous candida infection to occur due to a decreased number of IL-17 producing T cells. As a cell population related to immunity and infection, IL-17 producing T cells are critical components of the protective response against candidiasis despite their detrimental pro-inflammatory effect on autoimmune disorders. Another consideration is the co-secretion of cytokines in the micro-environment, which determines their potential action in various diseases.
Here are some limitations to this study. The first limitation of the study was the small size of the patients studied. Immunodeficiency disorders are rare disorders, among which CMCs are few in frequency, and therefore, a low number of these patients were anticipated at the beginning. The other limitation was that the studied patients had initially undergone immunologic screening evaluations by other physicians. Therefore, both phagocytic and combined immunodeficiencies were excluded according to lab findings. There were also no recurrent systemic and/or opportunistic infections in the clinical course of the disease. Hence, the patients were referred to us with an already established diagnosis of CMC. The results of the first evaluations were not available at the time of reporting the study (marked as NA in Table 2).
To conclude, in cases of recurrent candida infections, the initial assessment of IL-17 producing T cells may act as a predictor of an underlying primary immunodeficiency. In patients with low counts of IL-17 producing T cells, selecting a targeted panel of genetic tests may be more helpful in identifying the specific immunodeficiency disorder at an early stage than performing whole-exome sequence analysis.