1. Background
A significant proportion of healthcare-associated infections is ascribed to surgical site infections (SSIs), resulting in prolonged hospitalization, extensive antibiotic usage, and increased morbidity and mortality (1). Human and financial costs of SSIs are substantial, and from 1% (1) to 4.4% (2) of the patients undergoing surgery develop SSIs. Postoperative wound infection is a major cause of nosocomial infections and is responsible for 77% of postoperative deaths (3). The incidence rate of SSI varies depending on surgical preparations, as well as the wound’s, procedure’s, and patient’s characteristics (eg, surgical scrub, type of surgery, site of the wound, the extent of the trauma, and the presence or absence of comorbidities and underlying diseases) (3, 4). Wound infection can be prevented by proper antibiotic prophylaxis; however, wide-spectrum and/or inappropriate antibiotic usage can increase the risk of microbial resistance (5). Various guidelines have been developed for antibiotic prophylaxis in pediatric, neonatal, and adult surgery (6-8), but a review of the literature reveals that, in practice, antibiotic administration is not always based on guidelines. In a pediatric hospital in Spain (2018), antibiotic prophylaxis according to a standard protocol was fulfilled only in 41% of surgical cases (9). In 2011, a survey in Boston on 246316 surgical/invasive procedures revealed that 40% of children received antibiotics before surgery with no indication (10). In another study, in addition to prescribing incorrect prophylactic antibiotics, the duration of antibiotic therapy was also prolonged (11)
2. Objectives
In this study, we aimed to determine and compare the rate of SSI during a 6-month period before and after the implementation of an antibiotic monitoring program in the pediatric surgery ward of Mofid Children’s Hospital.
3. Methods
This quasi-experimental study was performed in Mofid Children’s Hospital, a tertiary care pediatric referral center. The rate of post-surgical infection was compared between two groups of children, one month to 15 years old, admitted during two time periods (Apr to Sep 2019 and Oct 2019 to Mar 2020).
All children with surgical indications referred during the study were included. Patients with shock (with or without sepsis) who underwent emergency surgery were excluded. Participants were selected by the convenience sampling method. In this study, there were two time periods: 1-The first six months in which prophylactic antibiotics were prescribed before surgery based on the traditional method, and 2-The second six months in which preoperative prophylactic antibiotics were administered according to the CDC guideline. In the first group, the patients received routine prophylaxis with broad-spectrum antibiotics, and in the second group, narrow-spectrum antibiotics in accordance with CDC guidelines were prescribed (6). The two groups were compared in terms of postoperative infections. Surgical site infection was defined as wound infection within 30 days (or 90 days if a prosthetic material was implanted) of surgery (12). In both groups, wound infections occurring during hospitalization and/or 30 days after surgery or up to 90 days after device insertion were documented. Patients with primary and secondary immunodeficiencies) diabetes, malnutrition, uremia, chemotherapy) were excluded from the study. Different types of wounds were also compared between the two groups, including:
A: Clean: No inflammation; the surgery not involving the respiratory, gastrointestinal, and/or genitourinary tract.
B: Clean/contaminated: The respiratory, gastrointestinal, and/or genitourinary tract is involved in the surgery, but a sterile technique was met with no contamination.
C: Contaminated: Major break in the sterile technique (ie, acute, non-purulent inflammation or gross spillage from the gastrointestinal tract).
D: Dirty or infected: The viscera are perforated, or there is an acute infection with pus during the operation, and/or delayed treated traumatic wounds, fecal contamination, or the presence of devitalized tissues (13). Information about wound infections in patients was kept confidential and not shared with other colleagues and patients. The study was approved under the ethical code of IR.SBMU.MSP.REC.1399.097. Patient information was entered in and analyzed by SPSS software.
4. Results
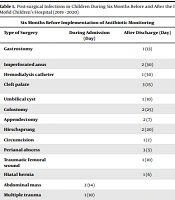
Two groups of surgical patients were studied for post-surgical infections. The first group (operated during Apr-Sep 2019) included 4380 cases (75% male), and the second group (hospitalized during Oct 2019–Mar 2020) encompassed 3650 cases (65% male). The age range was between 1 month and 15 years. The rate of infections was 31/4380 in the first group and 22/3650 in the second group (P-value = 0.3365), showing no significant difference as evidenced by the chi-square test (Table 1). The clinical manifestations of post-surgical systemic infections were fever, fever with vomiting, meningitis, and symptoms of urinary tract infections, and post-surgical wound infections were characterized by the discharge and redness of the surgical site. (Table 2) The type and number of the antibiotics prescribed for patients, before and after the implementation of the perioperative narrow-spectrum antibiotic prophylaxis protocol, according to the type of the surgery, have been shown in Table 3. The rate of infection for different types of wounds (clean, clean/contaminated, contaminated, and dirty) was not significantly different, as analyzed by the Mann-Whitney U test, between the two groups (P-value = 0.336, Table 4).
| Six Months Before Implementation of Antibiotic Monitoring | Six Months After Implementation of Antibiotic Monitoring | ||||
|---|---|---|---|---|---|
| Type of Surgery | During Admission (Day) | After Discharge (Day) | Type of Surgery | During Admission (Day) | After Discharge (Day) |
| Gastrostomy | 1 (13) | Invagination and laparotomy | 1 (30) | ||
| Imperforated anus | 2 (30) | Csf shunt | 2 (50) | ||
| Hemodialysis catheter | 1 (30) | Visceral extrophy | 1 (6) | ||
| Cleft palate | 3 (15) | Extrusion od double catheter | 1 (10) | ||
| Umbilical cyst | 1 (10) | Hirschsprung | 1 (7) | ||
| Colostomy | 2 (25) | Meatus stenosis | 2 (5) | ||
| Appendectomy | 2 (7) | Circumcision | 3 (3) | ||
| Hirschsprung | 2 (20) | Appendectomy | 2 (5) | ||
| Circumcision | 1 (2) | Ileostomy closure | 2 (7) | ||
| Perianal abscess | 3 (5) | Vesical extrophy | 1 (10) | ||
| Traumatic femoral wound | 1 (10) | Abdominal cyst | 1 (5) | ||
| Hiatal hernia | 1 (6) | Hepatic mass | 1 (15) | ||
| Abdominal mass | 2 (14) | Esophageal atresia | 2 (7) | ||
| Multiple trauma | 1 (10) | Multiple trauma | 2 (8) | ||
| Hydrocephalus | 2 (20) | ||||
| Gastric pull up | 1 (7) | ||||
| Total | 6 | 25 | 9 | 13 | |
Post-surgical Infections in Children During Six Months Before and After the Implementation of an Antibiotic Monitoring Plan at Admission and After Discharge in Mofid Children’s Hospital (2019 - 2020)
| Type of Infection | Six Months Before Implementation of Antibiotic Monitoring | Six Months After Implementation of Antibiotic Monitoring |
|---|---|---|
| During admission | ||
| Fever | 3 | 5 |
| Discharge and redness of surgical site | 1 | 1 |
| Fever and vomiting and meningitis | 2 | |
| Fever and discharge of surgical site | 2 | |
| Fever and urinary tract infection | 1 | |
| Total | 6 | 9 |
| After discharge | ||
| Discharge and redness of surgical site | 5 | 4 |
| Fever and vomiting and meningitis | 5 | 2 |
| Redness at surgical site | 2 | |
| Fever and discharge and redness at surgical site | 10 | 3 |
| Fever | 3 | 1 |
| Fever and vomiting | 1 | |
| Fever and urinary tract infection | 2 | |
| Total | 25 | 13 |
The Types of Post-surgical Infections in Children During Six Months Before and After the Implementation of an Antibiotic Monitoring Plan at Admission and After Discharge in Mofid Children’s Hospital (2019 - 2020)
| Type of Surgery | Six Months Before Implementation of Antibiotic Monitoring | Six Months After Implementation of Antibiotic Monitoring |
|---|---|---|
| Vats | Cefotaxime | Cefazolin |
| Lobectomy-pneumectomy | Cefazolin | Cefazolin |
| Tonsillectomy-lymph node biopsy | Cefazolin | Cefazolin |
| Port-insertion | Cefazolin | Cefazolin |
| Polypectomy with and without biopsy | Cefazolin- metronidazole | Cefazolin |
| Esophageal dilatation | Cefazolin | Cefazolin |
| Band ligation | Cefazolin | Cefazolin |
| Peg insertion | Cefazolin-cefotaxime-metronidazole | Cefazolin |
| Gall bladder surgery | Cefotaxime- metronidazole | Cefotaxime |
| Hernia repair | Cefazolin | - |
| Urologic surgery | Cefotaxime-metronidazole | Cefotaxime-metronidazole |
| Lymph node biopsy | Cefazolin | - |
| Mouth and tooth surgery | Cefotaxime-clindamycin | Cefotaxime-clindamycin |
| Cleft palate – clean wound | Cefotaxime | Cefazolin |
| Cleft lip – clean wound | Cefazolin | Cefazolin |
Types of Antibiotics Before and After the Implementation of an Antibiotic Monitoring Plan According to the Type of Surgery in Mofid Children’s Hospital (2019 - 2020)
| Type of Wounds | Six Months Before Implementation of Antibiotic Monitoring, No. (%) | Six Months After Implementation of Antibiotic Monitoring, No. (%) |
|---|---|---|
| Clean | 9 (30) | 5 (25) |
| Clean/contaminated | 9 (30) | 11 (50) |
| Contaminated | 2 (5) | 2 (5) |
| Dirty | 11 (35) | 4 (20) |
| Total | 31 (100) | 22 (100) |
Wound Types in Children at Six Months Before and After the Implementation of an Antibiotic Monitoring Plan at Admission and After Discharge in Mofid Children’s Hospital (2019 - 2020)
5. Discussion
The prevalence of surgical site infections differs between hospitals according to the type of the surgery and procedure. An overall rate of 9.9% (with a higher rate in public (13.4%) compared to private hospitals (6.5%)) was reported in Ethiopia in 2019 (12). Another study recorded the prevalence of 2.5% (2014) in orthopedic surgeries (14). The SSI rate was reported as 1% in 1830 surgical procedures in Italy (2017), and the incidence was lower in ENT procedures (1). In our study, the prevalence of surgical infections was 0.7% before the intervention and 0.6% after the implementation of the perioperative narrow-spectrum antibiotic prophylaxis protocol. The low rate of infections can be due to poor reporting and/or non-return or case mix of patients (ie, a higher number of surgical cases with a lower risk of secondary infections).
The majority of surgical cases in our study were males (75% and 65%, before and after the intervention, respectively). These results were similar to that reported by Al-Mulhim et al. and Halawi et al. (75% and 55.7% males, respectively) (14, 15). The higher number of males may be because boys seem to be more vulnerable to traumas; however, there is no information on the exact number of trauma cases in our patients.
The rate of surgery-associated wound infections decreases with the timely administration of appropriate prophylactic antibiotics. In many surgical centers, antibiotic prophylaxis either is compromised by prescribing wrong antibiotics, or the dose and time intervals are not according to recommended guidelines (15). In a study in India in 2019, a single bolus dose of intravenous antibiotics given before external dacryocystorhinostomy for acquired nasolacrimal duct obstruction to prevent post-surgical infections was as effective as oral antibiotics administered for five days (16). In a study in Taiwan in 2004, one-day versus three-day antibiotic prophylaxis with cefazolin showed no difference in preventing SSI up to one month after coronary artery bypass graft (13). In a meta-analysis conducted in 2018, there was no difference in the risk of wound infections between the adults receiving 1-day versus 5-day systemic antibiotic prophylaxis before clean-contaminated head and neck surgery (17). Although there are guidelines for antibiotic prophylaxis, care must be taken to ensure that these guidelines are followed precisely as recommended (6-8). In a meta-analysis study on 51627 patients with total joint arthroplasty, a comparison between preoperative single-dose vs. continuous (pre-and post-operative) antibiotic therapy retrieved a pool effect of 0.96, indicating no significant difference in efficiency (18). Similarly, in our study, we reduced the duration of antibiotics administered for perioperative prophylaxis, and the results revealed no effect on the rate of post-surgical infections compared to prolonged antibiotic administration. An Italian quasi-experimental 12-month study (ie, six months before and six months after the implementation of a care plan) was conducted to improve the accuracy of perioperative antibiotic prophylaxis (PAP) and reported an improvement in PAP using mono- and combination-antibiotic therapy (P = 0.02 and P = 0.004, respectively). The duration of antibiotic prophylaxis also decreased in this study (P < 0.001), and despite fewer days of antibiotic therapy and the use of narrow-spectrum antibiotics, no increase in treatment failure was recorded (P = 0.54) (19). Likewise, in our study, there was no change in the postoperative infection rate after reducing the number and dose of antibiotics in PAP.
In an Italian study, the percentages of clean wounds were 76.1% and 80.9% in the pre-and post-intervention periods, while there were no dirty wounds. However, in our study, the respective percentages were 30% and 25% for clean wounds and 35% and 20% for dirty wounds at pre-and post-intervention, respectively (19). In our study, the number of dirty wounds was higher due to the fact that our center was a referral hospital. In a study in USA in 2020 on colorectal procedures, the percentages of clean/contaminated, contaminated, and dirty wounds were 68%, 17%, and 15%, respectively, showing a higher value in the case of contaminated wounds compared to our study because of the nature of their procedure (20). To our knowledge, this is the first report on the rate of post-surgical infections in Mofid Hospital. Armin et al. studied the antimicrobial susceptibility patterns of six pathogens in Mofid Children’s Hospital and showed that all staphylococcus isolates were susceptible to vancomycin. In addition, the most effective antibiotics against Gram-negative bacteria were meropenem, amikacin, and Imipenem (21).
To address a limitation of this study, it is notable that there is a possibility that a ratio of postoperative infections, especially those occurring after discharge, may have remained unreported. The number of elective surgeries has probably decreased since the onset of the COVID-19 pandemic, but we do not have documented information on this. Because all patients in this study were screened after the onset of the COVID-19 pandemic, the presence of the pandemic could not have altered the results. Another limitation of this study was the lack of data on the duration of antibiotic therapy in the first group. In our protocol, antibiotics were not prescribed after surgery in most cases. However, due to the lack of documented information, it was not possible to compare the two groups in terms of the duration of antibiotic administration. The importance of this study lies in the fact that it is the first study that compares the rate of post-surgical infections before and after the implementation of a standard perioperative prophylactic antibiotic protocol in Iran.
5.1. Conclusions
The implementation of the perioperative narrow-spectrum antibiotic prophylaxis protocol was associated with no increase in the rate of wound infections after surgery as compared to broad-spectrum antibiotic administration for variable durations. It is recommended to implement the perioperative narrow-spectrum antibiotic prophylaxis plan under the supervision and follow-up of specialists in hospitals.