1. Background
Acute respiratory infection (ARI) causes about four million deaths in children under five years of age each year. It is one of the main causes of hospitalization and admission to intensive care in pediatrics. According to the World Health Organization (WHO), it is estimated that 2 - 3% of children under two years of age in low- and middle-income countries have had severe pneumonia requiring hospitalization, and ARI mortality rates fin these countries range from 60 to 100 cases per 1,000 children under the age of five (1).
In children under two years of age, viruses are the main etiological agents of ARI. Respiratory syncytial virus (RSV) has been considered to be the most common germ, and it usually affects children who have risk factors such as prematurity, heart disease, or bronchopulmonary dysplasia (2). A meta-analysis recently reported that, in 2005, between 66,000 and 160,000 children under five years of age died of RSV infection or complications directly related to RSV infection (2). The majority of these deaths occurred in developing countries. However, in the United States, deaths due to RSV infection are relatively low; in those under two years old, the mortality rate was between 3 and 4 per 10,000 hospital admissions (1, 2). The majority of these deaths were associated with prolonged hospital stays and at least one comorbidity.
Other viruses such as the rhinovirus/enterovirus (RV/EV) complex are thought to commonly cause uncomplicated acute upper respiratory infections that are usually self-limiting and have a benign course. It has recently been noted that this virus, from the genus Enterovirus and family Picornaviridae, can invade the lower respiratory tract and cause complicated disease in children (3, 4). Additionally, this virus has been associated with a higher frequency of bacterial coinfection and the presence of complicated pneumonia, accounting for up to 14% of acute respiratory distress syndrome (ARDS) in children living in high-income countries (3).
In low- and middle-income countries, there is no registry for RV/EV complex infection, its natural course, or associated risk factors. In these countries, the difficulty in accessing health services, chronic non-communicable diseases, and social inequality, frequently make ARI a major cause of both morbidity and mortality (1). Given that RSV is one of the viruses with the highest morbidity and mortality in children, it is important to compare the impact of RV/EV complex infection with that of this virus in order to understand the real burden of the disease in children and its possible associated factors.
2. Objectives
In this study, we intend to describe the main characteristics of hospitalized patients with ARI due to the RV/EV complex, the risk factors associated with severe infection, and their clinical course compared to RSV infections in children.
3. Methods
A retrospective descriptive study was carried out on children from one month to 18-years-old hospitalized in a university hospital in Bogotá, Colombia (Fundación Cardioinfantil-Instituto de Cardiología). A consecutive sampling of all patients who underwent multiple RT-PCR tests (FilmArray® BioMériux) and who were hospitalized for ARI on the regular hospital floor or in pediatric intensive care between October 2015 and December 2019 was performed. According to the institutional protocol, children with ARI who are going to be hospitalized should have an RT-PCR taken.
Post-transplant surgery patients who underwent multiple respiratory RT-PCR tests without having symptoms or a diagnosis of ARI were excluded. The Fundación Cardioinfantil-IC Ethics Committee approved this study with approval number PM-35-2020, and it was carried out following the ethical recommendations of the Declaration of Helsinki.
The information was taken from the electronic charts of hospitalized children who had undergone multiple RT-PCRs (using the Type IA10-2003 Zeptometrix 0810161CF technique). All the information was entered in a database created for this study, to which only the principal investigators had access.
The respiratory multiplex RT-PCR was ordered by the attending physician, according to his/her clinical judgment, for children with symptoms or a clinical picture suggestive of upper or lower respiratory tract infection. A respiratory therapy specialist took samples from nasopharyngeal aspirates, and they were sent to the central laboratory via the pneumatic tube system for rapid processing (within 30 minutes of collection), according to the institutional standards and protocols. The sample was taken using a flexible swab introduced along the length of the nasal septum, right above the nasal floor, until resistance was encountered. The swab was rotated against the nasopharyngeal mucosa for 10 seconds and withdrawn gently. Subsequently, the swab was placed in the transport medium, and the applicator stick was broken at the indicated level, using a minimum sample volume of 300 µL. The RT-PCR detects three bacteria (mycoplasma, Bordetella pertussis, and chlamydia) and 17 viruses (RSV, parainfluenza (1-4), influenza A/H3, influenza B, influenza A (H1, H1-2009), metapneumovirus, herpes virus, coronavirus OC43, coronavirus NL63, and adenovirus), with a sensitivity and specificity of 95% and 99%, respectively (5). It is currently considered the gold standard for these microorganisms (6).
Acute respiratory infections were defined as the group of respiratory tract diseases caused by various microorganisms such as viruses and bacteria, which began abruptly and lasted for less than two weeks (1). Severe respiratory infection was assessed in terms of the need for mechanical ventilation or transfer to intensive care, as well as the length of stay in the hospital. Acute respiratory infection and severe ARI groups were included. The clinical manifestations were classified into different diagnoses such as rhinopharyngitis, laryngitis, croup, bronchiolitis, tracheitis, pneumonia, or ARDS, according to the criteria of the attending physician. The data on RSV were recorded during the same period.
The outcome of unsatisfactory clinical behavior during RV/EV infection was defined as the requirement for an intensive care unit and/or mechanical ventilation, and the length of ventilatory support and hospital stay in pediatric patients hospitalized for ARI. Inpatient deaths from any cause in children with a positive RV/EV test were considered.
Severe ARI was defined as any high flow oxygen therapy including a fraction of inspired oxygen of more than 40%, the need for a high-flow nasal cannula, the use of non-invasive mechanical ventilation, the need for non-invasive mechanical ventilation, invasive mechanical ventilation, or the need for any vasoactive support. Patients whose viral infection was detected more than 48 hours after admission and who did not have symptoms on admission were considered to have a nosocomial infection. Viral coinfection was established when two or more respiratory viruses were isolated from the same sample using the same technique. In addition, bacterial coinfection was established by the presence of positive cultures (blood or tracheal secretion cultures) or the presence of procalcitonin > 0.5 μg/L.
3.1. Statistical Analysis
Descriptive statistics were used for demographic variables and a measure of central tendency. For continuous variables, the type of distribution was evaluated using the Shapiro Wilk normality test. Absolute and relative frequencies were reported for qualitative variables. Severity was assessed through a bivariate analysis using Student’s t-test for independent samples with normal distribution. A Mann-Whitney U test was used for non-normal distribution.
An exploratory bivariate analysis was performed to compare the two agents, RV/EV and RSV. Hospitalized and intensive care patients were analyzed. For the multivariate analysis, binary logistic regression was performed to control for confounding factors, mainly the severity of the disease, evaluated according to the PIM2 scale (7). This analysis aimed to establish the risk factors associated with severe RV/EV infection and determine their association with the outcomes of interest.
According to Smith and Wilson’s study (3), for a 95% confidence and 80% power, with a 5% probability of type I error and a 20% probability of type II error, assuming that the RV/EV infection group would have a 14% incidence of ARDS, with a non-exposed to exposed ratio of 0.33, at least 220 patients would be needed in the RV/EV group and 62 in the RSV group. Statistical analysis was performed using SPSS 25 (IBM 16), and a P ≤ 0.05 was considered statistically significant.
We tried to control biases with various strategies. To control for information bias, an exhaustive search of the electronic chart was carried out; if the information was lacking, other sources of information were searched, such as the data recorded in the clinical laboratory history or the nurses’ notes. The central laboratory’s RT-PCR sample collection and processing procedures were standardized. This research was carried out in a single university center that cares for all kinds of pathologies in the emergency room due to its complexity and size.
4. Results
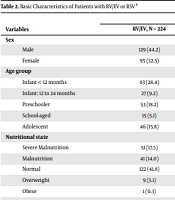
The RT-PCR was performed on 645 patients in the study period, of whom 224 were positive for RV/EV, and 68 were positive for RSV. The demographic characteristics of the patients are detailed in Table 1. The patients with RV/EV complex infection had a median age of 27 months (IQR: 8 - 70) and were predominantly male (54.8%; 123/224). This infection was seen more frequently in patients with solid organ transplantation (10.3%; 23/224) and prematurity (15.1%; 34/224) (Table 2).
| Patient Characteristics | N = 292 (%) | |
|---|---|---|
| Sex | Male | 160 (54.8) |
| Female | 132 (45.2) | |
| Age group | Infant < 12 months | 121 (41.4) |
| Infant 12 to 24 months | 35 (12.0) | |
| Preschooler | 68 (23.3) | |
| School-aged | 18 (6.2) | |
| Adolescent | 50 (17.1) | |
| Nutritional status | Severe malnutrition | 56 (19.2) |
| Malnutrition | 52 (18.2) | |
| Normal | 171 (58.6) | |
| Overweight | 11 (3.8) | |
| Obesity | 1 (0.3) | |
| Comorbidities | Kidney disease | 29 (9.9) |
| Liver disease | 40 (13.7) | |
| Heart disease | 58 (19.9) | |
| Prematurity | 33 (11.3) | |
| BPD | 25 (8.6) | |
| Metabolic disease | 20 (6.8) | |
| Transplant | 33 (11.3) | |
| Primary immunodeficiency | 35 (12.0) | |
| Neoplasm | 42 (14.4) | |
| Other comorbidities | Autoimmune | 24 (8.2) |
| Dermatologic | 8 (2.7) | |
| Hepatobiliar | 11 (3.8) | |
| Neoplasm | 6 (2.1) | |
| Asthma | 16 (5.5) | |
| CMV infection | 7 (2.4) | |
| Pulmonary hypertension | 9 (3.1) | |
| EBV infection | 2 (0.7) | |
| Symptoms on admission | Cough | 135 (46.2) |
| Dyspnea | 110 (37.7) | |
| Gastrointestinal | 45 (15.4) | |
| Neurological | 12 (4.1) | |
| Temperature (°C) (IQR) | 37.7 (35.6 - 40.1) | |
| Systolic blood pressure (IQR) | 96.9 (14 - 90) | |
| Base excess (mmol/L) (IQR) | 5.2 (2.6 - 6.0) | |
| Pa/Fi (IQR) | 292 (137.7 - 250.0) | |
| PIM 2 (IQR) | 0.9 (0.7 - 2.8) | |
| Total mortality | 13 (4.5) | |
| Cause of death | ARDS | 1 (7.7) |
| Septic shock | 3 (23.1) | |
| Multiple organ failure | 1 (7.7) | |
| Hypoxemic respiratory failure | 1 (7.7) | |
| Severe sequelae-DNR | 1 (7.7) | |
| Bradycardia and asystole | 1 (7.7) | |
| Refractory shock | 5 (38.5) |
Abbreviations: BPD, bronchopulmonary dysplasia; CMV, cytomegalovirus; EBV, Epstein-Barr virus; oC, degrees Celsius; FI02, inspired oxygen fraction; Pa/Fi, ratio of arterial pressure of oxygen and fraction of inspired oxygen; PIM, pediatric index mortality.
| Variables | Etiological Agent | P-Value | |
|---|---|---|---|
| RV/EV, N = 224 | RSV, N = 68 | ||
| Sex | 0.11 | ||
| Male | 129 (44.2) | 31 (10.6) | |
| Female | 95 (32.5) | 37 (12.7) | |
| Age group | 0.021 | ||
| Infant < 12 months | 83 (28.4) | 38 (13) | |
| Infant: 12 to 24 months | 27 (9.2) | 8 (2.7) | |
| Preschooler | 53 (18.2) | 15 (5.1) | |
| School-aged | 15 (5.1) | 3 (1.0) | |
| Adolescent | 46 (15.8) | 4 (1.4) | |
| Nutritional state | 0.05 | ||
| Severe malnutrition | 51 (17.5) | 5 (1.7) | |
| Malnutrition | 41 (14.0) | 12 (4.1) | |
| Normal | 122 (41.8) | 49 (16.8) | |
| Overweight | 9 (3.1) | 2 (0.7) | |
| Obese | 1 (0.3) | 0 (0) | |
| Diagnosis | < 0.001 | ||
| Croup | 7 (2.4) | 2 (0.7) | |
| Bronchiolitis | 32 (11.0) | 27 (9.2) | |
| Pneumonia | 82 (28.1) | 32 (11) | |
| Asthmatic crisis | 26 (8.9) | 0 (0) | |
| Recurrent wheezing | 8 (2.7) | 2 (0.7) | |
| ARDS | 32 (11.0) | 3 (1.0) | |
| Rhinopharinygitis | 28 (9.6) | 2 (0.7) | |
| Tracheitis | 2 (0.7) | 0 (0.0) | |
| Other | 7 (2.4) | 0 (0.0) | |
| Type of infection | 0.48 | ||
| Nosocomial | 47 (16.1) | 17 (5.8) | |
| Community-acquired | 177 (60.6) | 51 (17.5) | |
| Viral coinfection | 0.05 | ||
| Yes | 55 (18.8) | 9 (3.1) | |
| Bacterial coinfection | 0.04 | ||
| Yes | 57 (19.5) | 26 (8.9) | |
| PCT | 0.09 | ||
| Positive | 71 (38.2) | 27 (14.5) | |
| MV | 0.53 | ||
| Yes | 125 (53.9) | 32 (13.8) | |
| Respiratory support type | 0.45 | ||
| Invasive | 57 (24.6) | 14 (6.0) | |
| Non-invasive | 10 (4.3) | 1 (0.4) | |
| HFNC | 58 (25.0) | 17 (7.3) | |
| CNC | 51 (22.0) | 18 (7.8) | |
| Venturi | 6 (2.6) | 0 (0.0) | |
Abbreviations: RV/EV, rhinovirus/enterovirus complex; RSV, respiratory syncytial virus; ARDS, acute respiratory distress syndrome; PCT, procalcitonin; MV, mechanical ventilation; HFCN, high-flow nasal cannula; CNC, conventional nasal cannula.
aValues are expressed as No. (%).
Most of the patients with RV/EV complex infection had a normal weight (58.6%); nevertheless, 19.2% of the cases had severe malnutrition, and obesity was observed in 11 (3.8%) patients. The predominant symptoms were cough (46.2%) and dyspnea (37.7%), with some gastrointestinal (15.4%) and neurological symptoms (4.1%) (Table 1).
The final diagnoses found in RV/EV and RSV patients were pneumonia in 158 (54.1%) cases, and bronchiolitis in 59 (20.2%) cases, 35 (12%) of whom developed ARDS. Rhinopharyngitis, asthmatic crises, and recurrent wheezing occurred in 30 (10.3%) children, and croup and tracheitis in 10 (12%) patients (Table 2).
Severe respiratory infection criteria were identified in 162 (55.5%) cases. The main viruses found to cause viral coinfection with the RV/EV complex were found in 64 cases, as described in Table 3.
| Variables | N = 292 (%) |
|---|---|
| Viral coinfection | |
| Yes | 63 (21.5) |
| No | 229 (78.4) |
| Total | 292 (100) |
| Second isolated virus | |
| Respiratory syncytial virus | 16 (34.1) |
| Adenovirus | 2 (4.2) |
| Human metapneumovirus | 4 (8.6) |
| Influenza A/H1 | 1 (2.2) |
| Influenza A/H31 | 2 (4.2) |
| Influenza A/H1-2009 | 2 (4.2) |
| Parainfluenza virus 1 | 2 (4.2) |
| Parainfluenza virus 3 | 12 (25.5) |
| Parainfluenza virus 4 | 2 (4.2) |
| Coronavirus 229E | 1 (2.2) |
| Total | 47 (100.0) |
| Third isolated virus | |
| Respiratory syncytial virus | 1 (33.3) |
| Parainfluenza virus 3 | 1 (33.3) |
| Coronavirus NL63 | 1 (33.3) |
| Total | 3 (100.0) |
Of the 292 patients with RV/EV and RSV isolations, 172 (58.9%) had to be transferred to the Pediatric Intensive Care Unit (PICU). The use of some type of device for supplemental oxygen support was needed in 79.5% of the cases, with mechanical ventilation required in 157 (53.8%) patients.
The patients with RV/EV who had to be transferred to the PICU were younger than those who did not need to be transferred [13 months (IQR: 4 - 49) vs. 30 months (IQR: 10 - 65) (P = 0.004)] (Table 4). The predominant symptoms of the patients transferred to the PICU were more severe from the beginning. More dyspnea was observed at the time of emergency admission among those who were transferred to the PICU versus those who continued in general hospitalization (64.5% vs. 24.4%; P < 0.001).
| Variables | Total, No. (%) | P-Value | ||
|---|---|---|---|---|
| ARDS | < 0.001 | |||
| Yes | 33 (19.2) | 2 (1.7) | 35 (12) | |
| No | 139 (80.8) | 118 (98.3) | 257 (88) | |
| Total | 172(100) | 120 (100) | 292 (100) | |
| Severe ARI | < 0.001 | |||
| Yes | 149 (86.6) | 13(10.8) | 162 (55.5) | |
| No | 23 (13.4) | 107 (89.2) | 130 (44.5) | |
| Total | 172 (100) | 120 (100) | 292 (100) | |
| Mortality | 0.005 | |||
| Yes | 13 (7.6) | 0 (0) | 13 (4.5) | |
| No | 159 (92.4) | 120 (100) | 279 (95.5) | |
| Total | 172 (100) | 120 (100) | 292 (100) | |
Abbreviations: ARDS, acute respiratory distress syndrome; ARI, acute respiratory infection.
The most common comorbidities in the population admitted to the PICU were heart disease (24.4%), liver disease (15.1%), prematurity (15.1%), and bronchopulmonary dysplasia (13.4%). Other comorbidities included primary immunodeficiency (12.8%), transplantation (9.9%), metabolic disease (8.7%), and kidney disease (8.1%). Some type of neoplasm was present in 12 (7.0%) patients in the PICU versus 30 (25%) patients not in the PICU (P ≤ 0.001). Pneumonia was the predominant cause of admission to the PICU, with 72 (41.9%) patients, followed by bronchiolitis with 18% and asthmatic crisis with 11%. Severe ARI was identified in 86.6% of the cases that were transferred to the PICU, but only in 10.8% of those who were not transferred (P = < 0.001).
An analysis of the characteristics of the population with RV/EV versus RSV showed that the first group was older (27 months, IQR: 8 - 70 vs. 11 months, IQR: 2 - 29; P < 0.001). At the time of admission, the risk of dying, according to the PIM 2 scale, for both viruses was less than 1% (P = 0.69). The length of stay in the PICU for both had a median of five days (IQR: 3 - 12) (IQ: 4 - 13) (P = 0.58), with the need for respiratory support reaching a median of four days (IQR: 2 - 8) and seven days (IQR: 4 - 11) (P = 0.001), between RV/EV versus RSV. The presence of RV/EV infection increased the risk of developing ARDS (OR: 3.6; 95% CI: 1.07 - 12.18; P = 0.03).
In patients with a solid organ transplant, RV/EV infection was more frequently observed than RSV, 10.3% versus 1.0% (OR: 3.35; 95% CI: 1 - 11.34; P = 0.04). Viral coinfection was identified in 55 (18.8%) patients with RV/EV infection versus nine (3.1%) patients with RSV (OR: 2.13; 95% CI: 1 - 4.58; P = 0.04). Bacterial coinfection was observed in 19.5% of patients with RV/EV infection versus 8.9% with RSV (OR: 0.55; 95% CI: 0.31 - 0.98; P = 0.04).
The need for PICU was more frequently seen in the group with RV/EV infection, with 47.3% versus 11.6% in RSV (P = 0.09). acute respiratory distress syndrome developed more frequently in the group with RV/EV infection than in children with RSV infection (11% vs. 1%; P = 0.003). When different confounding factors (mainly, the severity of the disease measured by the PIM2 scale) were controlled for, ARDS in patients infected by RV/EV was observed more frequently in those with heart disease, premature infants, and those with inborn errors of metabolism (Table 5). There were a total of 13 deaths in all groups (4.5%), with no differences between the RSV group (54%) and children with RV/EV infection (46%) (P = 0.3) (Table 6).
| B | Standard Error | Wald | gl | Sig. | Exp (B) | |
|---|---|---|---|---|---|---|
| Heart disease | 1.095 | 0.552 | 3.934 | 1 | 0.047 | 2.99 |
| Prematurity | 1.802 | 0.637 | 8.011 | 1 | 0.005 | 6.062 |
| Metabolic disease | 1.611 | 0.751 | 4.61 | 1 | 0.032 | 5.01 |
| Variables | Etiological Agent | P-Value | |||
|---|---|---|---|---|---|
| RV/EV | RSV | ||||
| Viral Coinfection | Viral Coinfection | ||||
| Yes | No | Yes | No | ||
| Heart disease | 0.78 | ||||
| Yes | 12 (0.04) | 34 (0.12) | 4 (0.01) | 8 (0.03) | |
| No | 43 (0.15) | 135 (0.46) | 5 (0.02) | 51 (0.17) | |
| Prematurity | 0.76 | ||||
| Yes | 6 (0.02) | 16 (0.05) | 1 (0.00) | 10 (0.03) | |
| No | 49 (0.17) | 153 (0.52) | 8 (0.03) | 49 (0.17) | |
| BPD | 0.55 | ||||
| Yes | 6 (0.02) | 14 (0.05) | 0 (0.00) | 5 (0.02) | |
| No | 49 (0.17) | 155 (0.53) | 9 (0.03) | 54 (0.18) | |
| Immunodeficiency | 0.96 | ||||
| Yes | 7 (0.02) | 22 (0.08) | 1 (0.00) | 5 (0.02) | |
| No | 48 (0.16) | 147 (0.50) | 8 (0.03) | 54 (0.18) | |
| Neoplasm | 0.27 | ||||
| Yes | 6 (0.02) | 29 (0.10) | 0 (0.00) | 7 (0.02) | |
| No | 49 (0.17) | 140 (0.48) | 9 (0.03) | 52 (0.18) | |
| ARDS | 0.61 | ||||
| Yes | 9 (0.03) | 23 (0.08) | 0 (0.00) | 3 (0.01) | |
| No | 46 (0.16) | 146 (0.50) | 9 (0.03) | 56 (0.19) | |
| Mechanical ventilation | 0.47 | ||||
| Yes | 33 (0.11) | 92 (0.32) | 5 (0.02) | 27 (0.09) | |
| No | 16 (0.05) | 41 (0.14) | 1 (0.00) | 17 (0.06) | |
| Mortality | 0.81 | ||||
| Yes | 2 (0.01) | 5 (0.02) | 0 (0.00) | 6 (0.02) | |
| No | 53 (0.18) | 164 (0.56) | 9 (0.03) | 53 (0.18) | |
Abbreviations: BPD, bronchopulmonary dysplasia, RV/EV, rhinovirus/enterovirus ; RSV, respiratory syncytial virus
aValues are expressed as No. (%).
5. Discussion
In this study of children with RV/EV complex infection, 47.3% were found to have severe disease that required transfer to pediatric intensive care. Children with comorbidities such as prematurity, heart disease, and inborn errors of metabolism were especially prone to severe disease. The clinical course frequently includes ARDS and mortality similar to RSV, which has traditionally been responsible for a significant burden of disease (8).
The study population’s median age was much lower than that reported in the literature in high-income countries. Spaeder et al., in a retrospective cohort study carried out in Baltimore with 519 patients, showed a median age of 2.7 years (very similar to what we found in our group) for those hospitalized for RV/EV with severe infection (9). In a study in New York with 155 children, Smith and Wilson found a median age of four years (3).
Vásconez-García and Moyón-Constante have linked nutritional risk and the presence of worse outcomes and high mortality during severe viral respiratory infection in Latin American individuals with comorbidities (10, 11). However, in our population, malnutrition was observed in only 41%, a finding consistent with the dietary problems of low- and middle-income countries where the prevalence of chronic non-communicable diseases such as overweight is progressively increasing (12-15).
Traditionally, severe ARI has been described in patients with comorbidities. Tijerina-Tijerina et al., in a cross-sectional study in Mexico with 295 patients, described a higher rate of hospitalization in patients with prematurity (11) and bronchopulmonary dysplasia, relating it to the lack of coverage in vaccination, abandonment of breastfeeding, poverty, social commitment, and socioeconomic level (11, 16). In our population, we found that children hospitalized for RV/EV presented significant comorbidities and frequently needed to be transferred to intensive care. As mentioned previously, this virus has generally been thought to cause self-limited upper respiratory infection in most cases (17), but studies like ours show that, in risk groups, this virus can have similar behavior in terms of severity to others previously described, such as RSV.
Interestingly, we found that patients with RV/EV infection and heart disease, prematurity, and metabolic disease had a greater risk of ARDS, after controlling for confounding factors. Recently, Smith and Wilson (3) had similar findings in 22 patients with RV/EV infection, with this cohort showing that, in addition to the above risk factors, ARDS was more common in children with asthma exacerbations. These findings suggest that RV/EV infection may have an unsatisfactory course in risk groups and should be closely monitored.
We found that younger patients infected with RV/EV required a transfer to intensive care more often and had a longer hospital stay. Roeleveld et al., in high-income countries, found that RV/EV infection, regardless of comorbidities, was not associated with a longer length of hospital stay (18). The difference in our population can be explained by the greater difficulty in accessing health services in low- and middle-income countries, which often leads to late consultations. This is related to the significant frequency of ARDS seen in our series in children with RV/VE, which is higher than that observed with RSV.
Traditionally, RSV causes significant morbidity/mortality in children under two years of age. The study by Bianchini et al. shows that this virus is the second leading cause of infant mortality, with clinical manifestations and severe complications including ARDS (19). Having seen these results with RV/EV infection, it is striking that this germ had a more aggressive behavior, with a higher frequency of ARDS and viral coinfection and a greater need for respiratory support. This has not been described before in middle-income countries. We consider that it is important to look further into the pathophysiological mechanisms that explain this more severe behavior of RV/EV.
Messacar et al. found that pediatric patients admitted with the RV/EV complex had the same probability of requiring admission to the PICU or mechanical ventilation as those with influenza virus, which has historically been thought to produce more severe infections, with greater morbidity (20, 21). In a previous publication by our group, the RV/EV complex was described as the most frequent etiological agent (30%), followed by RSV (19%), parainfluenza (7.4%), and adenovirus (5.7%). Influenza only accounted for 1.2% of the total RT-PCRs taken (22). In his series, Messacar et al. found that only 18% of the patients with RV/EV required a transfer to intensive care, while 60% of our patients were transferred (20). Socioeconomic factors, comorbidities, and limitation of access to health services can explain this situation.
Even though it has been considered to be a minor infection, the risk of developing ARDS was more frequent with the RV/EV complex than with RSV infection in our population. This is an important fact, and we must consider that children admitted to intensive care with RV/EV infection may have an unsatisfactory course and require more frequent and intense support than those with other viruses such as RSV (5). Comorbidities such as prematurity, heart disease, and inborn errors of metabolism may partially explain this evolution, but this implies that, in these risk groups, RV/EV infection cannot be considered to be a mild infection, and its possible complications must be taken into account (5).
We consider that our study has several limitations. In the first place, it is the experience of a single university center that only evaluated children who, due to their severity, required hospitalization. This may be an explanation of why children with some comorbidities may require more transfers to intensive care. However, RV/EV infection was more severe and frequent in patients with comorbidities than RSV was in the same group. Another limitation of our study is that we did not have a control group with a negative RT-PCR and ARI that required a transfer to intensive care. However, when compared with RSV, we observed that the RV/EV behavior was more severe and had a more aggressive natural course. Additionally, the commercial brand of RT/PCR did not allow us to differentially evaluate the enterovirus and rhinovirus to understand the differences between these viruses from the same family (21). In general, they are analyzed together in most of the tests available commercially today.
5.1. Conclusions
Respiratory infection due to RV/EV in children can frequently have a severe course that requires management in intensive care. When compared to RSV, this virus is more frequently associated with the development of ARDS, especially in risk groups such as those with prematurity, heart disease, or inborn errors of metabolism. It is important to consider RV/EV as a virus that can have an unsatisfactory natural course equal to or more severe than other viruses that affect the respiratory tract in children.