1. Context
Coronaviruses are the largest RNA viruses associated with some ocular manifestations (1). There are some reports about the ocular manifestations of coronaviruses in humans. Different ocular tissues are involved in coronavirus-associated infectious diseases. The ductal connection between the upper respiratory tract and eye mucosa through the nasolacrimal duct can be an entrance to respiratory viruses, such as coronaviruses (2).
The coronavirus genome contains an encapsulated single-stranded positive-sense RNA. Coronaviruses are categorized in the Coronaviridae family and the Nidovirales order. The four-known genera of coronaviruses, including alpha-coronavirus, beta-coronavirus, gamma-coronavirus, and delta-coronavirus, are important pathogens in humans and other vertebrates. Coronaviruses can infect humans, livestock, birds, bats, mice, and other wildlife species (3). The seven known human-infecting species of coronaviruses are OC43, 229E, HKU1, NL63, MERS-CoV, SARS-CoV, and SARS-CoV-2. SARS-CoV-2 is the causative agent of coronavirus disease 2019 (COVID-19), first discovered in 2019 in Wuhan, China, and responsible for more than 410,000 mortalities (4). Studying these zoonotic viruses on animal models could be helpful in the future because these zoonotic viruses could transmit between animals and humans through mutations (5).
This article reviews the latest studies on the pathological relationship between coronaviruses and different eye parts, including the retina, conjunctiva, cornea, and uvea, in humans and animal models. Given the importance of investigating the pathogenesis and other routes of SARS-CoV-2 infection, especially in areas other than the respiratory tract, this report attempts to highlight the importance of eye infections caused by the virus, its role in maintaining the virus transmission chain, its impact on public health, and its effect on the COVID-19 pandemic.
2. Pathophysiology of Coronaviruses
The effects of coronaviruses are not well understood, but the members of this family seem to have the ability to infect and damage various tissues of the eye, including the retina, conjunctiva, cornea, and uvea. The effects of coronaviruses on the retina are limited to animal models and have not been observed in humans. The mouse hepatitis virus (MHV) is a retinotopic member of the coronavirus family (6). The retinal degeneration mouse model, known as experimental CoV retinopathy (ECOR) derived from MHV inoculation, was developed to investigate pathological processes within the retina. This model is also used to study the genetics and immune responses of the host, which may contribute to retinal disease. In this model, the condition is biphasic: (1) the initial phase, which includes inflammation; and (2) the late phase, which involves retinal degeneration (7, 8). ECOR is a degenerative disease of the retina caused by a virus affected by genetics and the host's immune response (9).
MHV-induced retinal infection begins in retinal pigment epithelium (RPE) and ganglion cells and then spreads to photoreceptors, Müller cells, and inner retinal layers optic nerve (10). There are several ways to inoculate MHV in mice. Pathological changes in the retina have only been observed in injections into the anterior chamber (AC) of the eye and the cerebral hemisphere. Inoculation of the virus into AC also results in long-term retinopathy. MHV has tropism in contrast to some retinal cells, including neural retina and RPE, due to specific MHV receptors on the surface of these cells (6, 11, 12). Conjunctivitis, or pink eye, is associated with coronavirus infection in humans and animals (13). In a 2003 study, the secretions and conjunctival cells of 17 patients infected with SARS-CoV were isolated, and the RT-PCR test on none of these 17 cases revealed a virus genome (14). The eye is indicated as a potential site for the transmission of the virus, and coronaviruses can spread through direct or indirect contact with mucosal membranes in the eye (15). Some studies showed that SARS-CoV-2 could cause eye complications such as viral conjunctivitis in the middle stage and not necessarily in the early stages of the disease (16). The pathological mechanism of SARS-CoV-2 infection in conjunctivitis is unclear because conjunctivitis can last more than a week. However, it is claimed that SARS-CoV-2-mediated conjunctivitis may be an abnormal autoimmune reaction. Loffredo et al.'s meta-analysis showed that conjunctivitis was more severe in COVID-19 and may be a prognostic hallmark for poor outcomes. Physicians should identify conjunctivitis as a possible sign of COVID-19 associated with a severe form of the disease (17).
Recent studies have shown that COVID-19 patients have SARS-CoV-2 viral particles in the conjunctival sac (18-20). In a study by Xia et al., the tear and conjunctival secretions of PCR-confirmed COVID-19 patients were collected twice (2 to 3-day intervals). This study showed that SARS-CoV-2 was present in tears and conjunctival secretions of only one COVID-19 patient with conjunctivitis, while the RT-PCR test of 29 other patients was negative (18). In one cohort study, 56 patients with COVID-19 were evaluated before and after the onset of COVID-19. This study showed that eye symptoms became more severe in about one out of four people after starting COVID-19. The eye symptoms appeared a few days before fever or respiratory symptoms in one out of ten patients (21). A 29-year-old woman with right eye conjunctivitis symptoms, photophobia, and clear watery discharge from the right eye was the first case of COVID-19 with keratoconjunctivitis as the main sign. The patient's conjunctival swab was also positive for SARS-CoV-2 (22). Wu et al.'s study was the first report on conjunctivitis in children with COVID-19. Two-year-old and 10-month-old infants had conjunctivitis and eyelid dermatitis on day 7 of the disease (23). SARS-CoV-2 productive infection and proliferation were observed in the conjunctiva and epithelial cells of intestinal carcinoma in a cell culture model. The proliferation of SARS-CoV-2 is more significant in the conjunctiva than in SARS-CoV (24).
Uveitis may appear as ocular pain, clinically shown in cats as partial protrusion of the third eyelid, squinting (blepharospasm), or tearing (epiphora). The most common form of uveitis in feline infectious peritonitis virus (FIPV) infection is bilateral granulomatous anterior uveitis. Ocular inflammation of FIP disease can gradually lead to panuveitis, an inflammation of all eye parts. Besides, FIP is the most common cause of uveitis in cats (25). Different ocular inflammatory manifestations have been observed in cats infected with FIPV infection, including pyogranulomatous or granulomatous inflammation throughout the uvea and in the sclera, conjunctiva, retina, and optic nerve (26).
3. Modeling of Retinal Pathogenesis of Coronaviruses
Inflammation plays a vital role in the pathogenesis of MHV in retinal contamination by this virus. This is because the infiltration of immune cells and the release of immune mediators in the retina and RPE lead to the destruction of photoreceptors and ganglion cells and the thinning of neuroretina (7). Various studies have determined the role of various immune cells in retinal damage caused by MHV (27-30). Interferon-gamma (IFN-γ) plays a vital role in limiting the proliferation and spread of the virus in the retina (27). Also, tumor necrosis factor (TNF)-a and soluble TNF receptor 2 (sTNFR2) are increased during MHV infection. These upregulations are associated with the progression of retinal degeneration (28). Also, MHV infection leads to specific CD4+ T cells activation against the MHV antigen and α-fodrin peptide. These CD4+ T cells lead to retinal degeneration caused by direct damage to retinal cells or infiltration of other immune cells into the retina (29). Infection with MHV leads to the upregulation of gene expression of FasL and granzyme B and activation of apoptosis in the retina where viral antigens and CD81+ T cells are present, eventually leading to the clearance of viral infection in the retina (30). Vascular endothelial growth factor (VEGF), which plays an essential role in some retinal diseases such as retinitis caused by the herpes simplex virus, does not play a role in the pathogenesis of MHV (30). The difference in the effect of the MHV virus on the retina in BALB/c mice and CD-I mice showed that host genetics was one of the critical factors in retinal pathology. The host could determine the level of virus replication and vertical transmission (31). The RNA of MHV can remain in the retina for 60 days, indicating a persistent viral infection.
Given that MHV infection in the mouse liver also shows a similar pattern of virus stability, it can be said that retinopathy caused by the virus is associated with persistent systemic infection (32). MHV can multiply in the retina, causing an acute sensory necrotizing disease of the retina and causing long-term sequelae. An essential feature of retinal damage is the absence of a detectable virus. The interphotoreceptor retinoid-binding protein (IRBP) level is decreased in the MHV-infected retina compared to the normal retina (33). A cell culture model in retinal-RPE cell co-cultures found that MHV induces stable viral infection, and cell-to-cell interactions of retinal-RPE cells could affect MHV infection in the retina (34). MHV is a potential therapeutic strategy for viral infections of the central nervous system and related diseases by transmitting retrograde axonal to the retina and targeting the spike virus protein interactions with axonal transport machinery (35).
The FIPV is another member of the coronavirus family with pathological effects on the retina. Ocular manifestations of FIPV choroiditis include retinal detachment and retinal vasculitis with perivascular cuffing by inflammatory cells (36). In some cases of FIPV-induced retinitis, glial fibrillary acidic protein (GFAP) expression is increased in molar cells, and in retinal detachment cases, these cells also proliferate. Both reactions indicate gliosis (37). Chorioretinitis, an inflammation of the choroid and retina, is also caused by vasculitis due to FIPV. This inflammation can lead to exudation from the choroidal blood vessels and, in more severe cases, can lead to retinal detachment (38). Compared to other inflammatory cells in cat-eye lesions, the predominance of B cells and plasma cells shows that the humoral immune response plays a vital role in eye inflammation in cats infected with FIPV. Unlike FIP-related lesions in other cat organs, macrophages are not the dominant inflammatory cells in FIPV-related eye lesions (37).
Despite the lack of reports of retinal involvement in human coronaviruses, given that the primary SARS-CoV-2 receptor, ACE2, is mainly expressed in the posterior tissues of the eye, such as the retina and RPE (39), it needs to do human studies in the future to establish the correlation between human SARS-CoV-2 and the retina.
4. Conjunctiva and Coronaviruses Infection
4.1. Human Conjunctiva and Coronaviruses Infection
Before the COVID-19 pandemic, there were limited studies of the effect of human coronaviruses on the conjunctiva. In some of these studies, eye involvement was not observed with SARS and MERS viruses (40-42). These studies found that the ocular effects of coronavirus infection in humans are not a common complication (40). Yuen et al. found no conjunctival effects, including intraocular pressure (IOP) and corticosteroid-related complications from SARS (41). In a 2003 study, the secretions and conjunctival cells of 17 patients infected with SARS-CoV were isolated, and the RT-PCR test on none of these 17 cases revealed a virus genome (14). The relatively small size of the specimen and taking only one specimen of tear swab and conjunctival scraping were among the limitations of this study. Despite studies that have ruled out the effect of coronaviruses on the eyes, other studies have shown the effects of these viruses on the eyes (43-46). The detection of RNA of SARS-CoV in the tears of three patients with SARS infection in the initial phases of the disease indicated the presence of the virus in the eyes (45). A 2014 study on 261 patients with MERS found that 2% of patients had conjunctivitis (46). Human Coronavirus NL63 (HcoV-NL63) was first detected in a seven-month-old child with symptoms of bronchiolitis and conjunctivitis (43). A 2005 retrospective study in France found that 17% of children with HcoV-NL63 had conjunctivitis. Other symptoms in children include fever, rhinitis, bronchiolitis, gastrointestinal problems, otitis, and pharyngitis (44).
4.2. Conjunctiva; A Zoonotic Location for Coronavirus Infection
In addition to studies on human specimens, several animal studies have been conducted. Evidence of the effect of animal coronaviruses on the eye suggests that conjunctivitis is present in cats (36, 47). In a study on cats infected with feline CoV (FcoV), 90% of infected cats had conjunctivitis, and the FcoV antigen could be detected in the conjunctiva of these cats (48). The conjunctiva of birds is one of the infection sites for the infectious bronchitis virus (IBV) at the beginning of infection (49). A study of camels infected with MERS-CoV found that the viral RNA can be detected at high concentrations in conjunctival swabs in the early phases of the disease (6). These few studies suggest some involvement of conjunctiva in animals by coronavirus infections.
4.3. Impact of the Conjunctiva on COVID-19
After Dr. Wang's national expert panel on pneumonia was diagnosed with conjunctivitis during a visit to Wuhan due to a lack of eye protection for SARS-CoV-2, attention was drawn to studying the ocular effects of SARS-CoV-2 (50). Despite numerous studies over the past few months on the eye effects of SARS-CoV-2, due to differences in the results of various studies, it has been difficult to conclude the relationship between SARS-CoV-2 and conjunctiva. Clinical evidence and laboratory results in the study of Liu and Sun showed that the conjunctiva was not a preferred tissue for SARS-CoV-2 and may rarely be infected by SARS-CoV-2. Also, the conjunctiva is not the main entrance to respiratory infections (51). A study by Peng and Zhou found that due to the absence of SARS‐CoV-2 RNA in tears and conjunctival secretions of COVID-19 patients without conjunctivitis, the virus cannot reproduce in the conjunctival epithelium and is less likely to be transmitted through the conjunctiva (52).
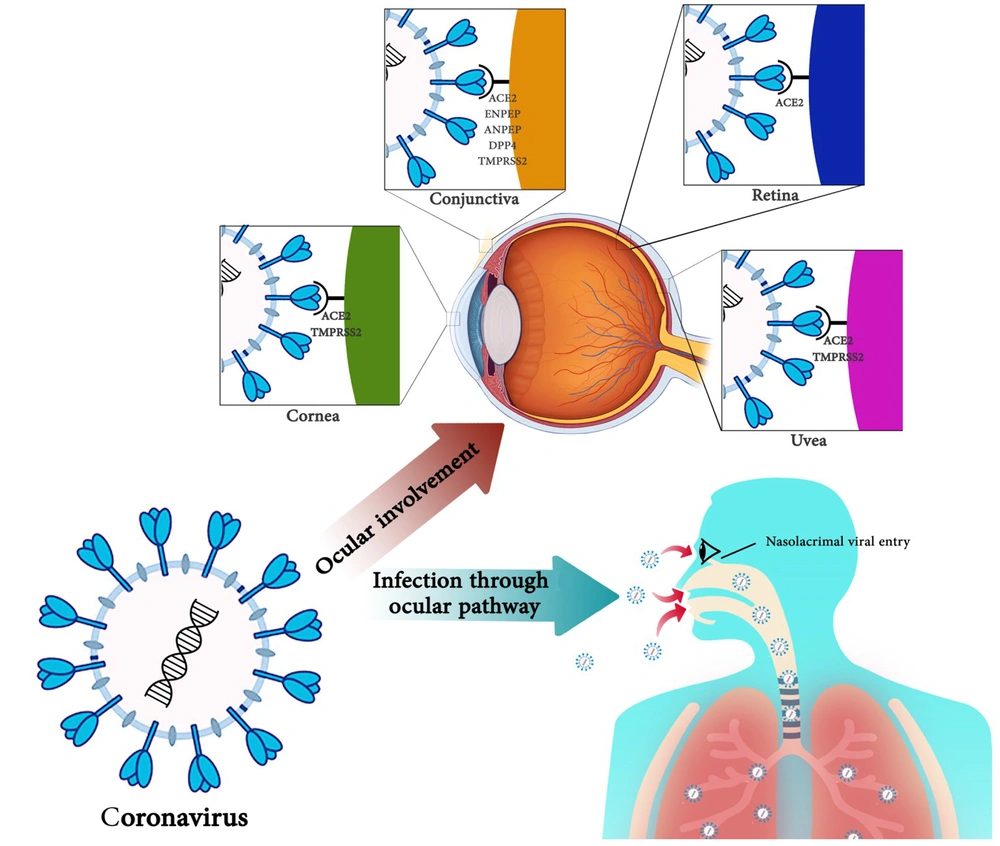
For this reason, detecting SARS-CoV-2 RNA may be a random event in tears, and conjunctival secretions in COVID-19 patients, and the virus may not be associated with conjunctivitis (52). A study conducted in Singapore on tear samples of 17 patients did not show a positive RT‑PCR test for SARS-CoV-2, and in terms of clinical symptoms, only one patient had conjunctival redness (53). Some studies suggest that the incidence of conjunctivitis is very low in patients with COVID-19 due to the low rate of expression of SARS-CoV-2 receptors in the conjunctiva (54). Also, high lactoferrin concentrations in tears prevent viral binding to sulfate heparan (55). Although it is stated that the primary SARS-CoV-2 receptor, ACE2, is not expressed in the conjunctival (39, 56) and co-mediators of SARS-CoV-2 entrance such as ENPEP, ANPEP, DPP4, and TMPRSS2 do not express stability in conjunctival tissues (56), there is some initial evidence for ACE2 expression and serine protease TMPRSS2 on conjunctival cells (44). In addition, conjunctival cells exhibit a similar expression of the TMPRSS2 gene to A549 cells (human lung cells) (57). These studies show ACE2 likely has little expression in the conjunctival tissue.
There are many studies of clinical evidence of conjunctivitis in COVID-19 patients or SARS-CoV-2 laboratory findings in conjunctival tears and secretions. Conjunctivitis can be a manifestation of COVID-19 (58). A nurse in the emergency department of Tongji hospital in Wuhan, China, with symptoms of conjunctivitis, went to the ophthalmology department of Tongji hospital. Both conjunctival and oropharyngeal swabs tested positive for SARS-CoV-2. Based on clinical manifestations, chest images, and laboratory findings, the patient was diagnosed with COVID-19 and acute viral conjunctivitis (16, 27). In a study by Wu et al., conjunctivitis symptoms were observed in one-third of SARS-CoV-2-infected patients, and this ocular complication was more common in people with more severe diseases. Also, the RNA of the virus was detected in conjunctival swab samples (59). SARS-CoV-2 can cause inflammation of the conjunctiva, line the inner part of the eyelid, cover the white part of the eye, and cause redness and itching (60). A retrospective study by Guan et al. reported a low incidence of conjunctivitis (< 1.0%) in COVID-19 patients (61). The study by Chen et al. found that of 534 patients with COVID-19, 112 (20.97%) had dry eyes, 68 had blurred vision (12.73%), and 63 had foreign body sensations (11.80%). Also, 25 (4.68%) patients had conjunctival congestion. Conjunctival congestion is one of the ocular symptoms associated with COVID-19 and may have clinical diagnostic value (62). In a study by Hong et al. on 56 COVID-19 patients, 15 (27%) had symptoms of eye surface irritation, two (~ 4%) had conjunctivitis, and SARS-CoV-2 was identified only in the eye of one patient with RT-PCR. They suggested that eye symptoms are relatively common in COVID-19 and may appear just before the onset of respiratory symptoms (21).
The presence of SARS-CoV-2 RNA over time in conjunctival specimens of a COVID-19 patient was first reported in a study by Zhang et al. The patient had eye problems with COVID-19 13 days after the onset of the disease, and RNA of SARS-CoV-2 could be detected in the patient's conjunctival sac samples on the 13th, 14th, and 17th days of the disease (16). The shedding pattern of SARS-CoV-2 in conjunctival specimens may differ from those of SARS-CoV and MERS-CoV. Viral RNA has been present in the patient's conjunctival sacs for at least five days, and its level in conjunctival specimens is lower than in respiratory samples (6). A study by Hu et al. on COVID-19 reported that despite the lack of conjunctival congestion or conjunctivitis, the virus was positive in the tear samples and conjunctival secretions (63). In Liang and Wu's study, with real-time PCR, SARS-CoV-2 RNA was positive in the conjunctival sac secretion of one out of 37 COVID-19 patients. Twelve of 37 COVID-19 patients had severe disease, and the rest had mild disease. Only three patients had conjunctival congestion symptoms (64). In a study by Zhang et al. on 72 COVID-19 patients, only two (2.78%) patients had conjunctivitis, and only one RT-PCR test (1.39%) of 72 patients was positive for SARS-CoV-2 in ocular secretions (16). In a study by Casalino et al., the first European confirmed case of COVID-19 with primary conjunctivitis symptoms was reported. A 65-year-old man was referred to the hospital with fever, discharged without fever, cough, and other symptoms, and diagnosed with conjunctivitis. He went to the emergency room two days later with symptoms of fever and shortness of breath, and a dry cough. His COVID-19 disease was confirmed by RT-PCR (65). Thus, COVID-19 disease can begin with conjunctivitis and appear as typical symptoms (66).
Regarding the association between SARS-CoV-2 and eye disease, two critical issues are essential: First, the effect of SARS-COV-2 on the eye, and second, SARS-CoV-2 takes advantage of the eye as a route to reach the respiratory system. In the above, we have looked at different studies of the effects of SARS-CoV-2 on the eye, but there is another more critical link between the eye and COVID-19 disease; ocular infection is a possible alternative route for SARS-CoV-2 transmission (16, 24, 50, 58). ACE2 expression can be detected at the eye level, including the conjunctiva and cornea, and provide the potential for eye entry for SARS-CoV-2 (67). Due to the ocular surface connection to the respiratory tract through the nasal passages, the virus may enter the respiratory tract through the ocular surface (68). The proposed mechanism of SARS-CoV-2 infection through the conjunctiva can drain respiratory droplets with tears into the nasolacrimal duct and respiratory tract, respectively (54). In the cynomolgus macaque animal model in Deng et al.'s study, SARS-CoV-2 was detected in conjunctival swabs only on the first day after conjunctival inoculation and in nasal and throat swabs from day one to day seven after conjunctival inoculation. This study showed that the conjunctiva is a potential portal for SARS-CoV-2 transmission. Viral load can be detected in several tissues associated with the nasolacrimal system, especially in the conjunctiva, lacrimal gland, nasal cavity, and throat, and shows a strong bridge between the eye and the respiratory tract. Mucosal membranes and unprotected eyes increase the risk of SARS-CoV-2 transmission. Eye contact with SARS-CoV-2 droplets or infected hands can infect the conjunctiva, and even if the virus does not cause eye problems, it reaches the respiratory tract through the eye and leads to respiratory tract infections (69). Therefore, eye protection equipment, such as glasses or shields, can somewhat reduce the risk of COVID-19. A study of patients with SARS-CoV found that unprotected eye contact was associated with transmitting the disease to healthy hygiene staff (70). As a result of a study on the effect of SARS-CoV-2 on the eye, it can be concluded that due to the low expression of the main receptor of SARS-CoV-2, i.e., ACE2, and its auxiliary receptors (57, 68) in the conjunctiva, the possibility of eye involvement is low in people at risk of COVID-19. This does not mean that the conjunctiva is not involved in COVID-19, and a review of these studies clearly shows that this organ is involved in SARS-CoV-2, albeit to a lesser extent.
5. Corona vs Cornea
Given that the cornea has an acceptable expression of ACE2 and TMPRSS2 genes compared to lung tissue, the relationship between coronaviruses and cornea requires further investigation (71). No human studies have been conducted to investigate the link between corneas and coronaviruses, and animal studies are limited. The cornea has a higher potential for SARS-CoV-2 infection than the conjunctiva. It is shown that SARS-CoV-2 receptors were more expressed in the cornea than in the conjunctiva. All of the rat tissues collected in this study could express ACE2. The ACE2 gene was higher in the corneal and liver tissues than in the lung, heart, kidney, and brain tissues, which had a moderate expression.
On the other hand, the expression of Tmprss2 is stable in the tissues of the cornea, lungs, liver, kidneys, and brain, and the cornea and liver have a more robust expression. Tmprss2 expression was much lower in the heart, spleen, and brain than in lung tissue. Measuring ACE2 and Tmprss2 expression in other eye tissues, including the iris, lens, retina, and optic nerve, showed that SARS-CoV-2 receptors were not expressed in these tissues (57). The human cornea and conjunctiva of ACE2, a SARS-CoV-2 receptor, could theoretically bind to and infect the two tissues (72, 73). In a study by Sun et al., who studied in-silico expression of ACE2 in humans and other mammals, it was found that ACE2 was expressed in corneal epithelial cells (72). A case report by Cheema et al. is the first report of a corneal-related conflict in human coronaviruses. In this study, a 29-year-old woman with COVID-19 with keratoconjunctivitis and mild respiratory symptoms, without fever, referred to an ophthalmologist, and corneal findings evolution and redness of the eye with watery discharge were identified through repeated patient visits. Because corneas are one of the localizations of coronavirus receptors, it is necessary to study coronavirus effects on them. These studies are more critical in corneal transplantation to prevent SARS-CoV-2 transmission through transplantation (22).
In addition to SARS-CoV-1 and SARS-CoV-2 receptors, CD209, a MERS-CoV receptor, can be found in human corneal dendritic cells (73). Complications of coronavirus have been reported in domestic cats (37, 74). After the cat became involved with FIPV in the opacity area of the cornea and the multifocal posterior synechia with dyscoria areas, superficial vascularization was observed. In this study, corneal lesions were consistent with corneal abscesses or neoplasia. The left cornea showed a dorsolateral 2-mm-diameter focal area of turbidity, and the corneal stroma contained a similar inflammatory infiltrate and formed a pyogranulomatous nodule (74). In feline corneas with feline infectious peritonitis, there were inflammatory cells, including lymphocytes, plasma cells, and macrophages, and inflammatory infiltration was observed mainly in the middle and deep layers of the corneal stroma (37).
6. Correlation Between Coronaviruses and Uvea
There have been no reports of human coronaviruses on the uvea so far. Studies in cats have reported the highest effect of coronavirus on the uvea. Diagnosis and treatment of anterior and posterior uveitis are the most critical factors in dealing with animals with FIP ocular manifestations (38). In the study of Felten et al., uveitis and neurological symptoms were more common in cats suffering from FIP without body cavity effusions. In this study, three cats with FIP underwent eye changes post-mortem examination. Twenty-two of the 26 cats had FIP effusions. According to uveitis, two cats had post-mortem changes (25). Clinical findings after euthanasia of a young hospitalized cat with fever, unilateral anterior uveitis, and respiratory distress indicated uveitis, pleural and mild abdominal effusion, and granulomas in the kidneys and skin (75).
While FIPV is the most common cause of uveitis in cats, the FIPV serological test is one of the applications in diagnosing domestic cats with uveitis (76). Cytological analysis of aqueous humor facilitates the diagnosis of anterior uveitis in dogs (77). Increased IL-6 activity in aqueous humor in cats with uveitis may be necessary for the clinical diagnosis of the disease (78).
In ferrets, systemic coronavirus infection closely resembles FIP in domestic cats and can present with anterior uveitis, chorioretinitis, optic neuritis, and retinal detachment. Systemic coronavirus disease should be differentiated as anterior uveitis in ferrets, similar to FIP in domestic cats (74). Although no reports deal with the correlation between human coronaviruses and uvea, studies on cell culture models or animal models could provide more information about the effects of coronaviruses on uvea.
7. Clinical Relevance
The overview of accessible literature suggests a very low transmission hazard through the ocular surface. This might also be due to the reality that although TMPRSS2 and ACE-2 receptors have been validated on corneal and conjunctival epithelial cells, the extensive range of mentioned receptors is negative in contrast to the respiratory cells. The receptor-binding capacity of the virus on ocular surface is low, due to the presence of lactoferrin in tears, which prevents the viral attachment to heparan sulfate proteoglycans (enrolled in binding to the ACE2 receptor). Also, serum IgA can play a protective role. The clinical and molecular relevance of coronaviruses with ocular diseases and manifestations is summarized in Table 1 (16, 44, 46, 49, 79).
| Disease | Tissue | Symptoms | Cells | Virus | Coronavirus Receptor Presence | References |
|---|---|---|---|---|---|---|
| Experimental CoV retinopathy (ECOR) | Retina | Inflammation, and retinal degeneration | Retinal pigment epithelium (RPE) and ganglion cells; Müller cells, inner retinal layers optic nerve | MHV | ACE2+ | (7) |
| Conjunctivitis | Conjunctiva | Pink eye photophobia and clear watery discharge | Non-keratinizing squamous epithelium, goblet (mucus) cells | SARS-CoV; SARS-CoV-2; MERS; Feline CoV; HCoV-NL63; IBV; | ACE2+/–; ENPEP–/+ ANPEP–/+; DPP4–/+; TMPRSS2–/+ | (16, 44, 46, 49, 79) |
| Retinitis | Retina | Choroidal blood vessels and, in more severe cases, can lead to retinal detachment | Müller cells | FIPV | ACE2+ | (36) |
| Uveitis | Uvea | Eye redness, eye pain, light sensitivity | - | FIPV | ACE-2 +/-TMPRSS2+/- | (25) |
| Corneal infection | Cornea | Redness of the eye with watery discharge | Human corneal dendritic cells; Corneal cells | FIPV; SARS-CoV-2 | ACE2 + TMPRSS2 + | (37, 80) |
Ocular toxicity is a side effect of drug-based treatments in COVID-19 patients. Long-term use of chloroquine and hydroxychloroquine can lead to retinal toxicity, but it has not been reported in short-term use of these drugs for COVID-19. Also, Lopinavir and Ritonavir can potentially lead to the reactivation of autoimmunity. Some ocular toxicities are reported for Ribavirin in COVID-19 patients, i.e., retinal vein occlusion, retinopathy, Vogt-Koyanagi-Harada (VKH) disease, non-arteritic ischemic optic neuropathy, and serous retinal detachment. Interferon may lead to Sjogren's syndrome, VKH, uveitis, optic neuropathy, retinopathy, conjunctivitis, and corneal ulcers. There are some reports regarding the cotton wool spots and retinal hemorrhage formation with the Tocilizumab medication. The receiving of systemic corticosteroids may lead to cataracts, central serous chorioretinopathy, and glaucoma. Central retinal vein occlusion is reported as another ocular cytotoxicity in receiving the intravenous immune globulin. These elements ought to be saved in concept by an ophthalmologist at some point in the records and examination of patients.
The ophthalmic features can occur at any stage of COVID-19 disease. The median time of neuro-ophthalmic manifestations from the time of symptoms or diagnosis of COVID-19 is five days. Also, this time for the ocular surface and anterior segment manifestations is 8.5 days, and for the posterior segment and orbital pathology is 12 days.
Using eye protection equipment by hygiene staff, especially ophthalmologists, is doubled. Because of the proximity of patients' noses and mouths and the possible exposure to tears that may contain the virus, ophthalmologists must be cautious when examining patients. Eye transmission is an essential occupational exposure route for medical staff. Many ophthalmologists involved in diagnosing and treating COVID-19 have been diagnosed with COVID-19. Given that triage by ophthalmology services may be the first SARS-CoV-2 transmission line and can be extended by contacting the aerosols with the conjunctiva, it is essential for health care providers to consider complete health protocols. The impacts of coronaviruses on the infection through the ocular pathway and eye involvement are summarized in Figure 1.
8. Conclusions
Before the COVID-19 pandemic, studies showed no association between coronaviruses and ocular manifestation in humans, but some showed this association in animals. Various coronavirus receptors are expressed on ocular tissue, which may be the reason for different ocular manifestations in COVID-19 patients and possible next epidemic/pandemic by the coronavirus family.