1. Background
Gastroenteritis is known as the leading cause of morbidity and mortality in children worldwide and diarrhea ranks second in the fatality rate of infants in the developing countries (1). Although diarrhea is prevalent in all ages, children are affected more due to rapid dehydration (2, 3). Diarrheal diseases cause 1 in 10 deaths in children under 5 years old (4). Poor sanitation, lack of safe drinking water, improper preparation of food and inadequate hygiene practices are the main risk factors of diarrhea (2). In recent years, numerous bacterial, viral, and parasitic agents are considered the main cause of gastroenteritis. Viral agents are the cause of the high rates of gastroenteritis, followed by bacterial and parasitic agents (1, 5). The human rotavirus is the most common etiologic agent of pediatric infections, which annually causes 125 million cases of diarrhea and more than 600 000 childhood deaths worldwide (6, 7). Bacteria account for 20% - 40% of diarrhea cases (8). Campylobacter jejuni, diarrheagenic Escherichia coli, non-typhoidal Salmonella spp. and Shigella spp. are frequently associated with diarrhea (9-11). Non-typhoidal Salmonella spp. annually causes 93,800,000 cases of gastroenteritis and 155,000 deaths globally (12, 13). Protozoan diarrhea is also distributed around the globe. Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum are the mostly reported protozoan pathogens that cause gastroenteritis (4, 5). Studies showed that approximately 100,000 deaths followed by gastroenteritis are related to protozoan species (12). Asymptomatic amebiasis is one of the most common complications of E. histolytica, but it is life threatening if parasite invades the intestinal mucosa. In this case, amebiasis may lead to dysentery, liver abscess, and even death. Amebiasis is regarded as the third cause of mortality after malaria and schistosomiasis (14). Identification of gastroenteritis-causing pathogens helps to establish surveillance systems and guides priorities to control the transmission of infections and prevents outbreaks (15).
2. Objectives
The current study aimed at elucidating the prevalence of bacterial, viral, and parasitic pathogens associated with children’s diarrhea in Ardebil, Iran.
3. Methods
The current retrospective study was conducted from December 2015 to June 2016 in Bu-Ali Hospital in Ardebil, Northwestern Iran. During the period, all stool specimens were collected from patients referred to Bu-Ali hospital as the study samples. Children within the age range of 2 months to 12 years with the symptoms of diarrhea, fever, vomiting, abdominal pain, and (in some cases) dehydration were selected. Children with bloody diarrhea were excluded from the study. The samples were tested for bacterial, viral, and parasitic agents. The viral samples were examined for rotavirus and adenovirus using the immunochromatographic strip method (Immunospark, Spain) (16, 17). Bacterial samples were plated onto eosin methylene blue (EMB) agar and Salmonella-Shigella agar (SS agar) media using standard microbiological techniques (18). After 24 hours of incubation at 37C°, bacterial cultures were identified by biochemical tests (9). Parasitic samples were examined by an immunofluorescence assay, microscopy, and trichrome staining. The enzyme-linked immunosorbent assay (ELISA) was used to differentiate Entamoeba histolytica from non-pathogenic Entamoebadispar.
4. Results
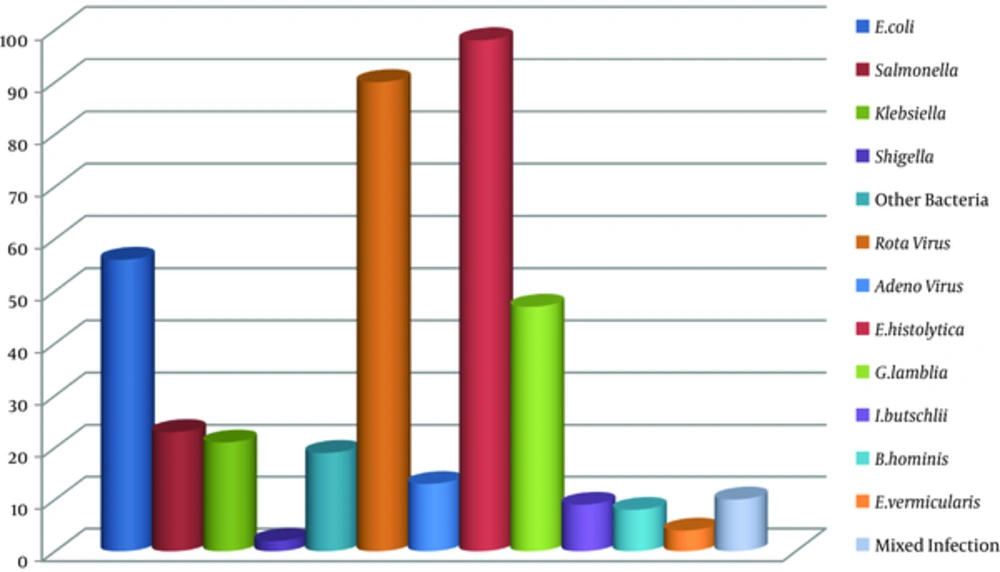
The study was conducted on 400 stool specimens of children within the age range of 2 months to 6 years. Of all samples, there were 100 (25%) viral, 121 (30.25%) bacterial, and 166 (41.5%) parasitic agents. Mixed infection was detected in 10 (2.5%) (2.5% confidence interval (CI): 1 - 4.5) cases (Figure 1). Out of 100 viral samples, the rotavirus constituted the largest proportion with 90 (22.5%) (21.75% CI: 17 - 26) cases, and the adenovirus made up the remaining 10 (3.25%) (3.25% CI: 1.9 - 5.4) cases. Of all bacterial pathogens, the most common one was Escherichia coli with 56 (14%) (14% CI: 10.94 - 17.74) cases, followed by non-typhoidal Salmonella spp., Klebsiella spp. and other bacteria with 23 (5.75%) (5.75% CI: 3 - 8), 21 (5.25%) (5.25% CI: 3.4 - 7.8) and 19 (4.75%) (3.75% CI: 2.29 - 6.09) cases, respectively. Parasitic pathogens were identified as Entamoeba histolytica with 98 (24.5%) (24.5% CI: 20.5 - 28.9), Giardia lamblia with 47 (11.75%) (11.75% CI: 8.9 - 15.2), Iodamoeba butschlii with 9 (2.25%) (2.25% CI: 1.19 - 4.2), Blastocystis hominis with 8 (2%) (2% CI: 0.01 - 3.9), and Enterobius vermicularis with 4 (1%) (1% CI: 0.03 - 2.0) cases, respectively. The results showed that parasitic agents had the largest proportion, followed by bacterial and viral agents in the study samples. Thus, the most prevalent viral, bacterial, and parasitic pathogens were rotavirus, E. coli, and E.histolytica.
5. Discussion
Gastrointestinal infections remain a major health problem despite the advances in infection control measures. Several studies are conducted to evaluate the prevalence of gastrointestinal pathogens in Iran. The current study found that 41.5% of samples were positive for parasites, 30.25% for bacteria, and 25.75% for viruses. Although bacterial and viral agents are traditionally considered the main causes of diarrhea, the current study indicated that protozoan pathogens were very important causes of diarrhea in this region. The current study found E. histolytica as the most prevalent parasitic agent. This result was in contrast with those reported by Sayyari et al. (19). The latter study reported G. lamblia as the major parasite in Iran, whereas the current study found it as the second most important causative agent. On the other hand, the current study result was compatible with the reports from Southwestern Iran (20). Pestehchian et al. and some other studies demonstrated a high prevalence of E. histolytica in the Southwest Iran (20, 21). In regards to the fact that intestinal protozoan infections are associated with lack of hygiene (22), sanitary measures should be improved in order to control infection and prevent fecal-oral transmission. The most effective preventive sanitary measures include: hand washing, proper food preparation, consumption of healthy water, and safe human waste disposal. Due to high costs of drugs and side effects followed by drug therapy, scientist suggest vaccination program as a favorable alternative (23). Since humans are the only known reservoir for E. histolytica, an effective vaccine can eradicate global E. histolytica infection (24, 25). In agreement with Zebardast et al., and some other studies, the current study results showed a higher prevalence of protozoan parasites compared with the helminthic agents (26). Similar to E. histolytica, E. coli infections are predominately due to lack of sanitary condition. In the current study, most viral infections were associated with the rotavirus, which was in accordance with several other studies from Iran (27-29). Previously conducted studies from other cities of Iran showed the large proportion of the rotavirus infection among children (27). Esteghamati et al., reported that, generally, more than half of all hospitalizations for severe diarrhea in Iran were associated with the rotavirus (30). A study in Tehran revealed a prevalence of 19% for rotavirus infections, while another study from Ahvaz showed the prevalence of 35% for rotavirus infection (31, 32). In another study, the rate of rotavirus positivity (42%) in Shiraz was higher than that of the current study results and the ones reported from other cities (33). It is considered that variety in the incidence of infection is associated with geographical conditions. Data from many other studies revealed the global seasonal occurrence of rotavirus infections (34, 35). Based on the preceding reports, rotavirus diarrhea frequently occurs during the winter months (34). The current study results supported the findings of the previously published studies and confirmed the abundance of rotavirus infections in cooler months (36). However, a report from Saudi Arabia showed the high incidence of rotavirus infection during dry and hot season (28). In addition, the seasonal pattern is not reported from countries with tropical climates such as Malaysia. With regard to the importance of the rotavirus, as the main cause of diarrhea in children (37), the rotavirus vaccination is a promising alternative to prevent the infection. The recommended vaccines by the world health organization (WHO) provide good protection and show a remarkable decrease in the seasonal prevalence of severe rotavirus infections. Rotavirus spread mainly occurs through fecal-oral route and contaminated hands (35); therefore, personal hygiene has a great impact on virus transmission. In the current study, similar to previous reports from Iran as well as other countries, the adenovirus had a lower rate compared with rotavirus (33). Also, there was no seasonal pattern for the adenovirus infection. Understanding the etiology of diarrhea advances in rapid and accurate diagnosis of microorganisms, and rational management certainly helps to prevent the outbreaks.
4.1. Conclusion
In conclusion, rotavirus, E. coli and E. histolytica were the most prevalent agents of diarrhea in children’s aged 2 month to 12 years in this study. Although detection of few micro organisms were considered in our study, the results indicate importance of hygiene improvement in this area. More specified molecular epidemiology studies with evaluating the most common etiologies will be helpful for a better understanding of the frequency of common infections and their transmission in this area.