1. Background
Like other countries in the world, working children live in Iran. The risky position of these children, including dysfunctional family, misery, and high accession of felony between family members and parents, cause social problems, such as rape and drug addiction in these high-risk populations (1). Working children live and grow without help, love, shelter, education, health, and medical facilities. These children live with severe deprivation and are exposed to a variety of physical and mental illnesses. One of the most important threats to working children is the high rate of sexual abuse, and as a result of these high-risk sexual relationships, the possibility of sexually transmitted infections (STIs) increases in this vulnerable group (2).
Because these children live in low-income families, they are forced to work on the streets and in unsuitable places as a source of income for families (3). The accurate number of working children is inaccessible, but approximately tens of millions of working children have been reported worldwide (4). In Iran, based on previous findings, it is estimated that there are about 20 000 to 2 000 000 working children (5). Working children face health problems (6), including anemia, malnutrition, infectious diseases (such as viral infections), and skin and gastrointestinal diseases (7). These children are more vulnerable to various infectious diseases, such as human papillomavirus (HPV), human immunodeficiency virus (HIV), viral hepatitis, and so on. Infection with these viruses threatens this group of children worldwide (8). Notwithstanding, a high rate of mortality and morbidity in working children has been shown to be due to numerous factors, such as inappropriate therapies, lack of facilities for access to medical care, nutritional deficiency, and lack of protection (9). On the other hand, the adversities of working children are growing at a considerable speed around the world (9). Anemia is a treatable disease that can cause mental health problems in these children (10). One of the essential elements for the growth of the body is calcium. With low levels of these essential elements, the health and development of children’s bodies are exposed to serious damage (11).
Various reasons have led to an increase in the number of working children around the world. The interaction between the community and working children is important (12). Parental disruption, including death, family breakdown, and divorce, has often resulted in poverty. As a result, these children become vulnerable (13).
In occult hepatitis B virus (HBV) infection (OBI), the genomic DNA of this virus is detectable in the blood or liver tissues. However, hepatitis B surface antigen (HBsAg) is not detectable in the serum or plasma (14). In occult hepatitis C virus (HCV) infection (OCI), HCV-RNA is detectable in peripheral blood mononuclear cells (PBMCs) and liver tissues in the infected patients. However, HCV-RNA is not detectable in the serum and is negative (15). The aim of this survey was to evaluate the prevalence of HCV, HBV, OCI, and OBI, as well as to determine the status of anemia and the level of calcium and phosphorus in working children in the first school years (Sobh-e Rooyesh School, Tehran, Iran). It is noteworthy that Iranian and Afghan working children attend Sobh-e Rooyesh School.
3. Methods
3.1. Study Population
A total of 370 consecutive Iranian and Afghan working children aged 6 - 15 years were enrolled in this cross-sectional survey from February 2018 to May 2019. All children attend the same school (Sobh-e Rooyesh School in Tehran, Iran). The current research was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran, in accordance with the Helsinki Declaration (code: IR.IUMS.FMD.REC.1398.541). After explaining the study procedure, informed consent was obtained from all the children’s parents or guardians.
3.2. Sample Collection
Blood samples, 6 mL, were taken from all children and transferred to a sterile tube containing anticoagulant EDTA. Plasma was separated using a centrifuge and frozen at -80°C until the extraction of viral RNA and DNA and serological assays. PBMC specimens were separated by Ficoll Hypaque density gradient centrifugation (Lympholyte HTM; Cedarlane, Hornby, Canada) and were washed 3 times with phosphate-buffered saline (PBS; pH 7.2 - 7.4). Then, they were resuspended in 200 µL of RNA preservative solution (RNAlater Ambion, Inc, Austin, TX, USA) and kept at -80°C for viral RNA and DNA extraction.
PBMC and plasma specimens from 5-HCV– and 5-HBV–infected individuals were used as positive controls. In addition, PBMC and plasma samples of 5 healthy individuals were used as a negative control of HCV and HBV infections.
3.3. Serologic Tests Using Enzyme-linked Immunosorbent Assays
HBV serological markers, including HBsAg, hepatitis B surface antibody (HBsAb), hepatitis B core antibody (HBcAb), and anti-HCV Abs, were analyzed using commercial enzyme-linked immunosorbent assay (ELISA) kits (DIA.PRO, Milano, Italy) according to the manufacturer’s protocol.
3.4. DNA/RNA Isolation
To detect the genomic HBV-DNA and HCV-RNA in the specimens of the children, viral DNA and RNA were extracted from the plasma and PBMC samples using a commercial kit (High Pure Viral Nucleic Acid [Roche Diagnostics GmbH, Mannheim, Germany]) according to the manufacturer’s instruction. Subsequently, the quantity and quality of the extracted RNA and DNA were tested using the NanoDrop spectrophotometer instrument (Thermo Fisher Scientific, Wilmington, USA).
3.5. Detection of HCV-RNA and HBV-DNA Using Real-Time Polymerase Chain Reaction
The genomic HCV-RNA and HBV-DNA were detected in plasma and PBMC specimens of the children by real-time polymerase chain reaction (PCR) (16, 17); also, as an internal control of these tests, the human β-globin gene was used as previously described in detail (18).
3.6. HCV Genotyping by Restriction Fragment Length Polymorphism and Sequencing
To confirm HCV infection and determine the genotype of the virus in a child who was positive for HCV infection in PBMCs, after the synthesis of cDNA, the 5'-untranslated (5'-UTR) region of this virus was amplified (19) by nested reverse transcriptase–PCR (RT-PCR); then, the HCV genotype was identified using the restriction fragment length polymorphism (RFLP) method (19). To confirm HCV genotyping with the RFLP method, the nonstructural protein 5B (NS5B) region of HCV was amplified using nested RT-PCR as previously described in detail (20). The PCR product (629 base pairs [bp]) of the second run of amplification was purified by a GenUP PCR/Gel Cleanup Kit (biotechrabbit GmbH, Berlin, Germany) according to the manufacturer’s protocol; then, it was sequenced bi-directionally using an ABI 3730 XL sequencer with the dye termination method. The nucleotide sequence of the NS5B region of HCV reported in the current study was submitted to the GenBank database with accession number MZ540985.
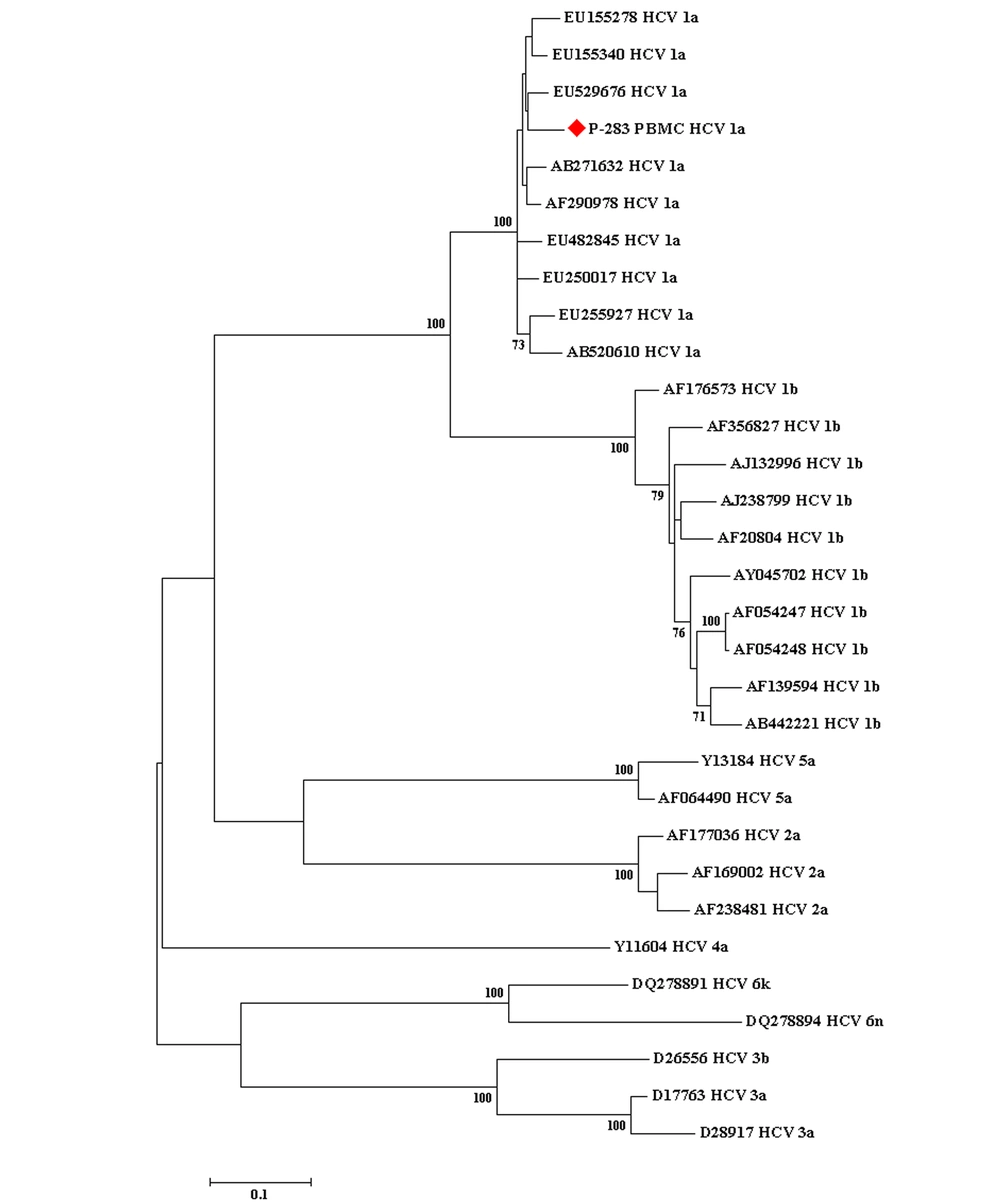
The sequences obtained from this research were aligned with the HCV reference sequences based on NS5B sequences (with various genotypes) that were retrieved from the GenBank database by the Crustal W method. The neighbor-joining method was performed for the construction of the phylogenetic tree. MEGA version 7.0 was used to draw the phylogenetic tree (Figure 1), and the statistical significance of the tree was assessed by the bootstrap method (1000 replicates).
The phylogenetic tree conducted based on the sequences of a conserved region of the nonstructural protein 5B gene of the hepatitis C virus obtained from the peripheral blood mononuclear cell sample of 1 working child with occult hepatitis C virus infection, as well as those corresponding to various hepatitis C virus reference sequences retrieved from the GenBank database. The bootstrap values equal to or greater than 70 obtained after 1000 replicates are illustrated in the nodes of the tree.
3.7. Statistical Analysis
The statistical analysis was performed using SPSS version 16 (SPSS Inc, Chicago, Ill, USA). For the determination of the quantitative variables’ normality, the Kolmogorov-Smirnov test was used. A continuous variable analysis was conducted using the Kruskal-Wallis and 1-way analysis of variance (ANOVA) tests. Chi-square and Fisher exact tests were used to evaluate the statistical differences between the 2 groups when appropriate. P-values less than 0.05 were considered statistically significant.
4. Results
A total of 370 working children (anti-HIV Ab/Ag negative) were included in the present cross-sectional survey from February 2018 to May 2019. The mean age of the participants was 10.1 ± 2.1 years (range, 6 - 15 years). Of the 370 subjects evaluated, 229 (61.9%) were male. All demographic and laboratory information of the studied children are summarized in Tables 1 and 2.
| Parameters | Male | Female | Total | P-Value |
|---|---|---|---|---|
| Demographic parameters | ||||
| No. of patients | 229 (61.9) | 141 (38.1) | 370 (100) | - |
| Nationality | 0.114 b | |||
| Iranian | 45 (19.7) | 20 (14.2) | 65 (17.6) | |
| Afghan | 184 (80.3) | 121 (85.8) | 305 (82.4) | |
| Age (y) | 10.4 ± 2.2 (7 - 15) | 9.5 ± 1.7 (6 - 13) | 10.1 ± 2.1 (6 - 15) | < 0.001 c, d |
| Laboratory data | ||||
| HCV Ab | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - |
| HCV RNA in plasma | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - |
| HCV RNA in PBMC1 | 1 (0.4) | 0.0 (0.0) | 1 (0.3) | 0.619 b |
| HBsAg | 2 (0.9) | 0.0 (0.0) | 2 (0.5) | 0.382 b |
| HBsAb | 80 (34.9) | 69 (48.9) | 149 (40.3) | 0.005 b, c |
| HBcAb | 2 (0.9) | 1 (0.7) | 3 (0.8) | 0.675 b |
| HBV DNA in plasma | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - |
| HBV DNA in PBMC3 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - |
| White blood cells | 6800 ± 1607 (3700 - 11000) | 6300 ± 1436 (4200 - 10000) | 6600 ± 1564 (3700 - 11000) | 0.002 c, d |
| Hemoglobin (g/dL) | 13.2 ± 0.9 (11 - 15) | 12.4 ± 1.1 (9.8 - 14) | 12.9 ± 1.1 (9.8 - 15) | < 0.001 c, d |
| Hemoglobin categorized | < 0.001 b, c | |||
| < 11.5 g/dL | 12.0 (5.2) | 34.0 (24.1) | 46 (12.4) | |
| ≥ 11.6 g/dL | 217.0 (94.8) | 107.0 (75.9) | 324 (87.6) | |
| Iron (mg/dL) | 73.8 ± 22.6 (32 - 120) | 74.7 ± 20.6 (18 - 118) | 74.1 ± 21.8 (18 - 120) | 0.735 e |
| Calcium (mg/dL) | 10.1 ± 0.6 (7.4 - 10.8) | 10.2 ± 0.2 (9.6 - 10.6) | 10.1 ± 0.5 (7.4 - 10.8) | 0.815 d |
| Calcium categorized | 0.008 b, c | |||
| < 8.8 mg/dL | 11.0 (4.8) | 0.0 (0.0) | 11 (3.0) | |
| ≥ 8.9 mg/dL | 218.0 (95.2) | 141.0 (100) | 359 (97.0) | |
| Phosphorus (mg/dL) | 4.8 ± 0.5 (3.6 - 5.9) | 4.9 ± 0.5 (3.3 - 5.8) | 4.9 ± 0.5 (3.3 - 5.9) | 0.114 e |
| Fasting blood sugar (mg/dL) | 84.7 ± 9.3 (65 - 115) | 87.3 ± 8.2 (68 - 107) | 85.7 ± 9.0 (65 - 115) | 0.006 c, e |
| Urea (mg/dL) | 22.6 ± 4.7 (13 - 35) | 22.1 ± 5.9 (13 - 38) | 22.4 ± 5.2 (13 - 38) | 0.150 d |
| Creatinine (mg/dL) | 0.6 ± 0.08 (0.5 - 0.9) | 0.5 ± 0.05 (0.5 - 0.7) | 0.6 ± 0.09 (0.5 - 0.9) | < 0.001 c, d |
| Uric acid (mg/dL) | 3.8 ± 1.1 (1.9 - 6.6) | 3.2 ± 0.63 (1.9 - 4.6) | 3.6 ± 0.99 (1.9 - 6.6) | < 0.001 c, d |
| Cholesterol (mg/dL) | 149 ± 25.7 (104 - 216) | 162 ± 20.9 (112 - 207) | 154.1 ± 24.8 (104 - 216) | < 0.001 c, e |
| Triglyceride (mg/dL) | 86.3± 43.5 (25 - 210) | 77.8 ± 41.0 (29 - 191) | 83.0 ± 42.5 (25 - 210) | 0.023 c, d |
| Level of education, grade | 0.002 c, f | |||
| First | 35 (15.3) | 22 (15.6) | 57 (15.4) | |
| Second | 45 (19.7) | 45 (31.9) | 90 (24.3) | |
| Third | 36 (15.7) | 33 (23.4) | 69 (18.6) | |
| Fourth | 35 (15.3) | 17 (12.1) | 52 (14.1) | |
| Fifth | 48 (21.0) | 11 (7.8) | 59 (15.9) | |
| Sixth | 30 (13.1) | 13 (9.2) | 43 (11.6) |
Abbreviation: PBMCs, peripheral blood mononuclear cells.
a All the continuous data were represented by the mean ± SD (for normally distributed data) or by the median and interquartile (for non-normally distributed data). Categorical data were presented by the No. (%).
b Fisher exact test
c Statistically significant
d Mann - Whitney U test
et-test
f Chi-square
| Parameters | Iranian Children | Afghan Children | Total | P-Value |
|---|---|---|---|---|
| Demographic parameters | ||||
| No. of patients | 65 (17.6) | 305 (82.4) | 370 (100) | - |
| Sex | 0.114 b | |||
| Male | 45 (69.2) | 184 (60.3) | 229 (61.9) | |
| Female | 20 (30.8) | 121 (39.7) | 141 (38.1) | |
| Age (y) | 9.1 ± 2.0 (6 - 13) | 10.3 ± 2.1 (6 - 15) | 10.1 ± 2.1 (6 - 15) | < 0.001 c, d |
| Laboratory data | ||||
| HCV Ab | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| HCV RNA in plasma | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| HCV RNA in PBMC1 | 0 (0.0) | 1 (0.3) | 1 (0.3) | 0.824 b |
| HBsAg | 0 (0.0) | 2 (0.7) | 2 (0.5) | 0.321 b |
| HBsAb | 27 (41.5) | 122 (40.0) | 149 (40.3) | 0.462 b |
| HBcAb | 1 (1.5) | 2 (0.7) | 3 (0.8) | 0.441 b |
| HBV DNA in plasma | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| HBV DNA in PBMC3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| White blood cells | 6756 ± 1796 (3700 - 10000) | 6603 ± 1512 (4200 - 11000) | 6600 ± 1564 (3700 - 11000) | 0.002 c, d |
| Hemoglobin (g/dL) | 13.0 ± 1.0 (11 - 14.7) | 12.9 ± 1.1 (9.8 - 15) | 12.9 ± 1.1 (9.8 - 15) | < 0.001 c, d |
| Hemoglobin categorized | 1.000 b | |||
| < 11.5 g/dL | 57 (87.7) | 38 (12.5) | 46 (12.4) | |
| ≥ 11.6 g/dL | 8 (12.3) | 267 (87.5) | 324 (87.6) | |
| Iron (mg/dL) | 80.1 ± 22.1 (32 - 111) | 72.9 ± 21.6 (18 - 120) | 74.1 ± 21.8 (18 - 120) | 74.1 ± 21.8 (18 - 120) |
| Calcium (mg/dL) | 10.1 ± 0.6 (8.8 - 10.8) | 10.1 ± 0.5 (7.4 - 10.6) | 10.1 ± 0.5 (7.4 - 10.8) | 10.1 ± 0.5 (7.4 - 10.8) |
| Calcium categorized | 0.005 b, c | |||
| < 8.8 mg/dL | 6 (9.2) | 5 (1.6) | 11 (3.0) | |
| ≥ 8.9 mg/dL | 59 (90.8) | 300 (98.4) | 359 (97.0) | |
| Phosphorus (mg/dL) | 4.9 ± 0.3 (4.5 - 5.5) | 4.6 ± 0.6 (3.3 - 5.9) | 4.9 ± 0.5 (3.3 - 5.9) | 0.006 c, e |
| Fasting blood sugar (mg/dL) | 83.6 ± 8.6 (68 - 104) | 86.1 ± 9.0 (65 - 115) | 85.7 ± 9.0 (65 - 115) | 0.039 c, e |
| Urea (mg/dL) | 19.8 ± 5.0 (13 - 29) | 23.0 ± 5.1 (15 - 38) | 22.4 ± 5.2 (13 - 38) | 0.150 d |
| Creatinine (mg/dL) | 0.59 ± 0.09 (0.5 - 0.9) | 0.6 ± 0.09 (0.5 - 0.9) | 0.6 ± 0.09 (0.5 - 0.9) | < 0.001 c, d |
| Uric acid (mg/dL) | 3.7 ± 0.5 (2.9 - 4.6) | 3.6 ± 1.1 (1.9 - 6.6) | 3.6 ± 0.99 (1.9 - 6.6) | < 0.001 c, d |
| Cholesterol (mg/dL) | 159.3 ± 27.8 (112 - 198) | 153.0± 24.2 (104 - 216) | 154.1 ± 24.8 (104 - 216) | 0.091 e |
| Triglyceride (mg/dL) | 76.1± 33.7 (25 - 171) | 84.5 ± 44.2 (29 - 216) | 83.0 ± 42.5 (25 - 210) | 0.023 c, d |
| Level of education, grade | 0.004 c, f | |||
| First | 20 (30.8) | 37 (12.1) | 57 (15.4) | |
| Second | 11 (16.9) | 79 (25.9) | 90 (24.3) | |
| Third | 14 (21.5) | 55 (18.2) | 69 (18.6) | |
| Fourth | 9 (13.8) | 43 (14.1) | 52 (14.1) | |
| Fifth | 6 (9.2) | 53 (17.4) | 59 (15.9) | |
| Sixth | 5 (7.7) | 38 (12.5) | 43 (11.6) |
Abbreviation: PBMCs, peripheral blood mononuclear cells
a All the continuous data were represented by the mean ± SD (for normally distributed data) or by the median and interquartile (for non-normally distributed data). Categorical data were presented by the No. (%).
b Fisher exact test
c Statistically significant
d Mann - Whitney U test
et-test
f Chi-square
Among 370 participants, the population of Iranian and Afghan nationalities was 65 (17.6%) and 305 (82.4%), respectively. Regarding Iranian nationality, 45 (19.7%) were male, and 20 (14.2%) were female. Regarding Afghan nationality, 184 (80.3%) were male, and 121 (85.8%) were female (Table 2).
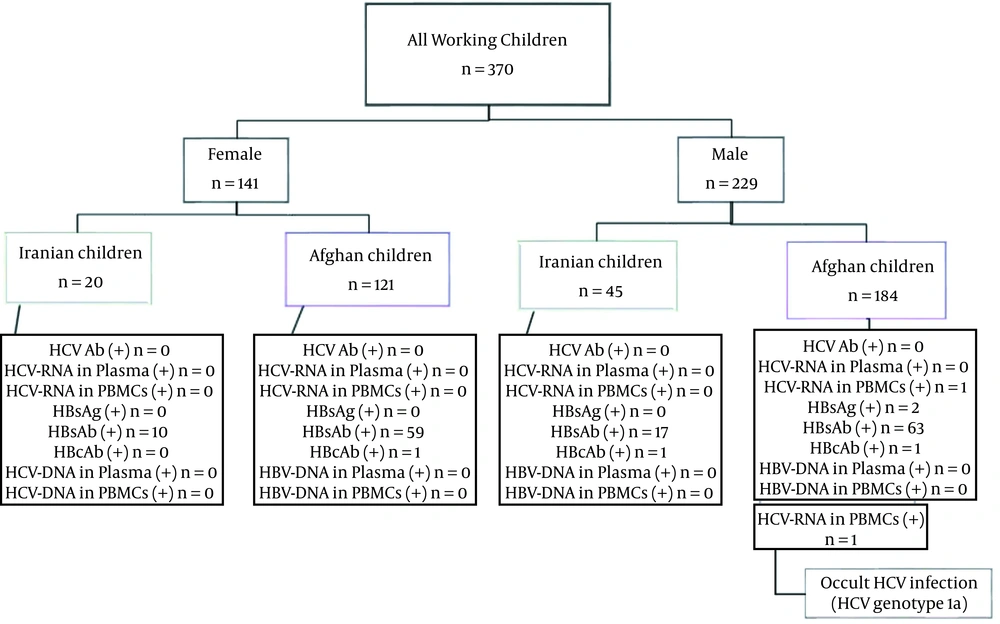
All of the children with a negative anti-HIV Ab/Ag test were evaluated for HCV and HBV infection. Detection of HCV-RNA in plasma and PBMC was conducted. Similarly, these assessments were performed to detect HBV-DNA in plasma and PBMC samples. None of the studied subjects had any detectable genomic DNA of HBV in plasma and PBMC samples. Therefore, the studied children were negative for HBV and OBI. In addition, none of these studied children had any detectable HCV-RNA in plasma. However, one (0.3%) of these studied children had detectable HCV-RNA in the PBMC sample. Thus, one of the children had OCI (Tables 1 and 3). The genotype of HCV detected in this child was subtype 1a.
In both Iranian and Afghan populations, the results of HCV Ab detection were negative. It is also noteworthy that 2 (0.54%) of the working children in this study were HBsAg positive; further, HBsAb was detected in Iranian (41.5%) and Afghan children (40.0%). Out of 149 (40.3%) children with positive HBsAb, 80 (53.7%) were male, and 69 (46.3%) were female. Further, positive HBcAb test results were observed in 3 (0.8%) participants. Out of 3 positive HBcAb participants, 2 (0.9%) and 1 (0.7%) were male and female, respectively. All information related to molecular and serological tests is summarized in Figure 2. All demographic and laboratory data of the working children with OCI, HBsAg, HBcAb, and so on are shown in Table 3.
| No. | Age/Gender | Nationality | ALT (IU/L) | AST (IU/L) | HCV-Ab | HCV-RNA in Plasma | HCV-Gen in Plasma | HCV-RNA in PBMCs | HCV-gen in PBMCs | HBs-Ag | HBs-Ab | HBc-Ab | HBe-Ag | HBe-Ab | HBV-DNA in Plasma | HBV-DNA in PBMCs | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 113 | 12/M | Afghan | 19 | 20 | - | - | - | - | - | + | + | + | - | - | - | - | - |
| 122 | 11/F | Afghan | 17 | 18 | - | - | - | - | - | - | + | + | - | - | - | - | - |
| 166 | 10/M | Afghan | 23 | 25 | - | - | - | - | - | + | - | - | - | - | - | - | - |
| 177 | 12/M | Iranian | 15 | 14 | - | - | - | - | - | - | - | + | - | - | - | - | Isolated HBcAb |
| 283 | 15/M | Afghan | 27 | 30 | - | - | - | + | 1a | - | - | - | - | - | - | - | OCI |
Abbreviations: M, Male; F, Female; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; gen, Genotype; PBMCs, peripheral blood mononuclear cells; OCI, occult HCV infection
In this research, no statistically significant association was found between gender and HBcAb, while a significant association was observed between gender and HBsAb (P = 0.005; Fisher exact test). In addition, no statistically significant association was found between gender and iron, calcium, phosphorus, and urea, while a significant association was observed between gender hemoglobin, creatinine, and uric acid (P < 0.001; Mann-Whitney U test), fasting blood sugar (P = 0.006; t-test), cholesterol (P < 0.001; t-test), and triglyceride (P = 0.023, Mann-Whitney U test).
Furthermore, no significant association was observed between nationality and HBsAb/HBcAb. In addition, no statistically significant association was found between nationality, calcium, and cholesterol. However, a significant association was observed between nationality and hemoglobin (P < 0.001; Mann-Whitney U test), iron (P = 0.015; t-test), phosphorus (P = 0.006, t-test), fasting blood sugar (P = 0.039; t-test), creatinine and uric acid (P < 0.001; Mann-Whitney U test), and triglyceride (P = 0.039; Mann-Whitney U test).
In addition, a significant association was observed between gender and education (P = 0.002; chi-square test). Moreover, a significant association was observed between nationality and education (P = 0.004; chi-square test). In this survey, a significant association was found between gender and categorized hemoglobin (P < 0.001; Fisher exact test) and categorized calcium (P = 0.008; Fisher exact test), as well as between nationality and categorized calcium (P = 0.005; Fisher exact test).
5. Discussion
There is little evidence on the prevalence of HBV and HCV infections in working children in Iran. According to our knowledge, this is the first research to evaluate the prevalence of OBI and OCI among this group of people. Therefore, the purpose of the current research was to detect the epidemiology of OBI, OCI, and HCV/HBV coinfection among working children. The present study was conducted on Iranian and Afghan nationalities. None of the studied participants had any detectable genomic HBV-DNA in plasma and PBMC specimens. Thus, studied children were negative for active HBV and OBI. Furthermore, none of the working children had any detectable HCV-RNA in plasma. It is noteworthy that one (0.3%) of these studied children had detectable HCV-RNA in the PBMC specimen. Therefore, one of the Afghan children had OCI, and he was infected with HCV genotype 1a.
Negative test results were reported for HCV Abs in both Iranian and Afghan populations. In addition, 2 Afghan children were positive for HBsAg. Therefore, the percentage of HBV infection in these children was 0.54%. Generally, positive anti-HBs (HBsAb) test results were detected in 149 (40.3%) participants. Of 149 (40.3%) children with positive HBsAb, 80 (34.9%) were male. In addition, positive HBcAb test results were detected in 3 participants. Out of 3 (0.8%) positive HBcAb participants, 2 (0.9%) and 1 (0.7%) participants were male and female, respectively. Thus, it appears that 0.8% of children may have been exposed to the hepatitis virus infection during their lifetime.
It is necessary to mention that among children, the normal range of white blood cell counts (WBCs) is 5000 to 10 000 per microliter of blood. Totally, the range of WBCs in the studied participants was between 3700 and 11000 cells. Low WBC count in these children leads to infection susceptibility (21). Iron deficiency and anemia are one of the major public health issues worldwide, particularly in developing countries (22). Totally, the range of hemoglobin and iron in the studied children was 9.8 - 15 gm/dL and 18 - 120 μmol/L, respectively. In this survey, a significant association was found between gender and categorized hemoglobin and calcium. The hemoglobin level was classified as below and above 11.5, and it was observed that the hemoglobin level was much lower in women than in men; it seems that women suffer from anemia (23). Due to anemia, it seems that these children should be treated for anemia. In addition, the calcium level was classified as below and above 8.8 (4), and it was observed that calcium levels were lower in men than in women; men seem to suffer from relative calcium deficiency than women.
Moreover, for both nationalities, serum calcium, phosphorus, fasting blood sugar, urea, creatinine, uric acid, cholesterol, and triglyceride were measured. Generally, after following these children, serum calcium level was 7.4 - 10.8 mg/dL. Therefore, approximately, a normal value was detected for these participants. Phosphorus is one of the essential minerals in the body required for teeth and bone health. In addition, it is also a critical element in muscle contraction and nerve signaling. Normal range of phosphorus in children is 4.0 - 7.0 mg/dL (24). In the present study, totally, the range of phosphorus was found to be 3.3 - 5.9 mg/dL.
Generally, HCV and HBV infections lead to chronic liver disease. According to the latest data, over 250 million individuals live with HBV infection; also, more than 70 million people live with HCV infection (25, 26). Various studies have been conducted on HBV and HCV infections worldwide. In Nanoro, HBsAg (0.8%) was reported in children (27). In Taiwan, out of 1510 preschool children, the prevalence of children infected with HBV was 15.9%, HBsAg-positive cases were 7.8%, and positive–anti-HBs (HBsAb) cases were 8.1% of the studied population (28). In Nigeria, a rate of 10% was reported for HBsAg-positive preschool children (29). In China, the OBI-positive rate was 3.1% (10/327), and the HBV-DNA rate was 14.1% (46/327) among HBV-vaccinated children with HBV-infected parents (30). In Japan, OBI (1.3%) was reported among immunized children with HBV carrier mothers (31). In Kuala Lumpur, anti-HCV (0.6%) was reported in children (32). In Vancouver, Canada, among street youth, the rate of HCV seropositivity was 10.6% (33). In the Afghan population, the rate of HBV and HCV infections was 1.9% and 1.1%, respectively (34). In Iran, HBV-DNA (21/75; 28%) was reported among immunized children with HBV-infected mothers (35), and also, among working children and street children, the rate of HBV and HCV infections was 1.7% and 2.6%, respectively (5). In another study from Iran, among street children, the prevalence of HCV, HBsAg, and HBsAb was 0.0%, 3%, and 15%, respectively (36), and it is also reported that among working children, negative results for HCV infection were reported. However, the rate of HBsAg was 0.59% (8). In Iran, among street children, HBsAg positive, HBsAb, HBcAb, and HCV-Ab were reported to be 3%, 26.6%, 8%, and 3.5%, respectively (37). However, the findings of the current research do not support the previous research (30, 31). The results are consistent with previous studies for HCV infection; however, they are completely different for HBsAg and HBsAb (36).
HCV is a hepatotropic virus; it should be noted that there are lines of evidence for replication and the presence of this virus in PBMCs (38). Although PBMCs are not the primary site of virus replication, some reports have emphasized the role of these cells as HCV reservoirs (39). Several studies have shown that the presence of the virus genome in extrahepatic reservoirs has significant effects on disease transmission and progression (39, 40). It is noteworthy that active replication of HCV occurs in the presence of a negative polarity strand; thus, the presence of a negative sense strand is an indicator of active replication of the virus. Although hepatocytes are the major site of HCV replication, the negative-strand RNA of the virus is also found in PBMCs (41); therefore, it can be concluded that HCV multiplies in these cells.
Castillo et al. reported a specific and unusual form of chronic HCV (OCI) (41). In this infection, the HCV genome in the liver or PBMC samples was detected in the absence of antibodies against the virus and the genomic HCV-RNA in plasma specimens (41). In this study, it was found that one of the children in this survey had OCI. To our knowledge, this is the first study to evaluate the prevalence of OCI in working children (0.3%). Therefore, the results of the current survey cannot be compared with another study. Nevertheless, we can compare the prevalence of this infection with other groups.
The presence of OCI has been observed in different populations around the world, for instance, in individuals with liver disease with unknown etiology in Spain (57.0%) (41), and in Iran (10.1%) (42), in people with HIV infection in Iran (9.2%) (43), and in Georgia (10%) (44). This infection has been diagnosed in individuals with high level of ALT (32%) in Iran (45), in hemodialysis patients in Thailand (18.2%) (46), and in Germany (0.25%) (47), in people with lymphoproliferative disorders in Iran (1.9%) (48), in Spain (13.3%), and in Egypt (20%) (49). Also, this viral infection has been detected in patients with beta thalassemia major in Iran (5.7 %) (50), and in another report from Iran (6.7%) (51), in Egyptian HCV infected patients who achieved sustained virologic response (SVR) to Sofosbuvir/Daclatasvir therapy (3.9%) (52). Therefore, the presence of this infection has been observed in various groups of people. However, it is important to consider that there are limited reports of the absence of this infection in different groups of people (53, 54). The current study found that the presence of OCI in working children is about 0.3%; thus, it seems that the possibility of the presence of this infection in working children should be considered. The genotype of HCV detected in the child with OCI was subtype 1a, which is the predominant subtype of this viral infection in Iran (20, 43, 55).
A limitation of the present study is that some parents of the studied children were not interested in their children entering this study; accordingly, they did not enter the present study. During the blood sampling, some of them did not cooperate and did not allow blood sampling; thus, they were not included in the current survey.
5.1. Conclusions
None of the studied children was HCV-RNA-/HBV-DNA positive synchronously. However, it is noteworthy that OCI was observed in these children with very low prevalence. Therefore, it seems that in addition to the routine experiments to detect different infectious diseases in this population, appropriate tests to diagnose OCI are informative and should be considered.