1. Context
Coronavirus 2019 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which appeared in Wuhan, Hubei, the People’s Republic of China, at the end of September 2019. Novel coronavirus disease 2019 (COVID-19) poses a serious medical challenge, as well as significant societal and economic consequences (1, 2). The disease is a global epidemic that affects 632,161,336 cases and causes 6,580,216 deaths in 237 countries, regions, or territories until October 21, 2022 (3). Although COVID-19 can affect all age groups, children or adolescents appear less susceptible to the infection (4). In addition, reports of severe forms of COVID-19 are rare in the pediatric population (5). Although the pandemic is not over and a new variant of concern (Omicron) and its sublineages BA.1, BA.2, BA.2.12.1, BA.4, and BA.5 cause considerable concern at the end of 2021, COVID-19 cases and hospitalizations are on the downslope, and deaths also continue to decrease, which promises the final days of the coronavirus pandemic (6).
2. Objectives
Our systematic review and meta-analysis aimed to determine the most common comorbidities, clinical signs and symptoms, imaging features, treatments, outcomes, and complications in the early phase of the COVID-19 pandemic. Our systematic review and meta-analysis compared adult and pediatric patients to identify the differences better.
3. Methods
3.1. Search Strategy
The study is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (7). Until June 4, 2020, systematic searches were conducted on the EMBASE, PubMed, Web of Science, Google Scholar, and Scopus databases to identify and retrieve studies on clinical symptoms, computed tomography (CT)-scans findings, and clinical outcomes in adults and children. The search strategy is shown in Supplementary File. In addition, we performed a manual search to find additional related studies.
3.2. Study Selection
The PICO format is considered and accordingly all children and adult cases with COVID-19 infection were included and compared for clinical symptoms, CT-scans findings, and clinical outcomes. Clinical signs and symptoms, CT-scan findings, supportive care, and patient outcomes were all investigated in observational and interventional studies on adults and children. The prevalence percentages and 95% confidence intervals were calculated by comparing different variables. Animal studies, as well as reviews and meta-analyses, were omitted. The meta-analysis rejected studies that did not provide sample sizes. Non-English articles have been removed as well.
3.3. Data Extraction and Quality Assessment
The full relevant data was investigated by M. V. and H. F. separately. A third researcher (A. A.) also investigated their accuracy. The following information is gathered using standardized forms: The first author’s name, publication year, nation, population characteristics, study design, sample size, comorbidity percentage, children and adult support, and therapies, as well as signs and symptoms. The Newcastle-Ottawa rating form was used to assess the studies’ quality. Selection (four items), comparability (one item), and outcome (three items) are all included in this assessment form. Three types of final scores were provided: Good (three or four stars in the selection domain, one or two stars in the comparability domain, and two or three stars in the outcome/exposure domain), fair (two stars in the selection domain, one or two stars in the comparability domain, and two or three stars in the outcome/exposure domain), and poor (no or one star in the outcome/exposure domain) (8). Table 1 represents the quality assessment results.
| Author | Selection | Comparability | Outcome | Total | Quality |
|---|---|---|---|---|---|
| Abrishami, A. | 3 | 1 | 2 | 6 | Good |
| Aggarwal, S. | 2 | 1 | 2 | 5 | Fair |
| Jianghong A. | 3 | 1 | 3 | 7 | Good |
| Ashraf, M. A. | 3 | 1 | 2 | 6 | Good |
| Benelli, G. | 3 | 1 | 2 | 6 | Good |
| Bhatraju, P. K. | 3 | 1 | 3 | 7 | Good |
| Bo, X. | 3 | 1 | 2 | 6 | Good |
| Cao, D. | 2 | 1 | 2 | 5 | Fair |
| Chen, J. | 3 | 1 | 2 | 6 | Good |
| Chen, N. | 3 | 1 | 2 | 6 | Good |
| Chen, S. | 2 | 1 | 2 | 5 | Fair |
| Colombi, D. | 3 | 1 | 3 | 7 | Good |
| Du, Y. | 3 | 1 | 2 | 6 | Good |
| Eghbali, A. | 2 | 1 | 2 | 5 | Fair |
| Haseli, S. | 3 | 1 | 2 | 6 | Good |
| He, W. | 3 | 1 | 2 | 6 | Good |
| Hu, L. | 3 | 1 | 2 | 6 | Good |
| Huang, K. S. | 3 | 1 | 2 | 6 | Good |
| Huang, C. | 2 | 1 | 2 | 5 | Fair |
| Jiang, X. | 2 | 1 | 2 | 5 | Fair |
| Luo, X. | 3 | 1 | 2 | 6 | Good |
| Ng, M. Y. | 3 | 1 | 2 | 6 | Good |
| Su, L. | 3 | 1 | 2 | 6 | Good |
| Tian, S. | 3 | 1 | 2 | 6 | Good |
| Wang, R. | 3 | 1 | 2 | 6 | Good |
| Xu, H. | 3 | 1 | 2 | 6 | Good |
| Yang, N. | 2 | 1 | 2 | 5 | Fair |
| Jiang-shan, L. | 3 | 1 | 2 | 6 | Good |
| Zhang, J. | 3 | 1 | 2 | 6 | Good |
| Chen, Y. | 3 | 1 | 2 | 6 | Good |
| Wang, L. | 3 | 1 | 3 | 7 | Good |
| Palaiodimos, L. | 3 | 1 | 3 | 7 | Good |
| Zhou, Y. | 3 | 1 | 3 | 7 | Good |
| Sun, H. | 3 | 1 | 3 | 7 | Good |
| Belhadjer, Z. | 3 | 1 | 2 | 6 | Good |
| Cummings, M. J. | 3 | 1 | 2 | 6 | Good |
| Fadel, R. | 3 | 1 | 2 | 6 | Good |
| Guan, W. | 3 | 1 | 3 | 7 | Good |
| Li, L. | 3 | 1 | 2 | 6 | Good |
| Ling, L. | 2 | 1 | 2 | 5 | Fair |
| Liu, T. | 2 | 1 | 2 | 5 | Fair |
| Pan, C. | 2 | 1 | 2 | 5 | Fair |
| Yan, Y. | 3 | 1 | 2 | 6 | Good |
| Yang, X. B. | 3 | 1 | 2 | 6 | Good |
| Zhang, G. | 3 | 1 | 2 | 6 | Good |
| Zhu, L. | 2 | 1 | 2 | 5 | Fair |
| Spinello, A. | 2 | 1 | 2 | 5 | Fair |
| Mayla Gabriela Silva, B. | 3 | 1 | 2 | 6 | Good |
| Cao, B. | 3 | 1 | 2 | 6 | Good |
| Chao, J. Y. | 3 | 1 | 2 | 6 | Good |
| Mahévas, M. | 3 | 1 | 2 | 6 | Good |
| Buckner, F. S. | 3 | 1 | 3 | 7 | Good |
| Chen, T. | 3 | 1 | 3 | 7 | Good |
| Docherty, A. B. | 3 | 1 | 2 | 6 | Good |
| Du, R. H. | 3 | 1 | 2 | 6 | Good |
| Hur, K. | 4 | 1 | 1 | 6 | Good |
| Inciardi, R. M. | 3 | 1 | 2 | 6 | Good |
| Israelsen, S. B. | 2 | 1 | 2 | 5 | Fair |
| Itelman, E. | 3 | 1 | 2 | 6 | Good |
| Jeong, E. K. | 3 | 1 | 2 | 6 | Good |
| Nikpouraghdam, M. | 3 | 2 | 2 | 7 | Good |
| Pedersen, H. P. | 3 | 1 | 2 | 6 | Good |
| Petrilli, C. M. | 3 | 1 | 2 | 6 | Good |
| Petrilli, C. M. | 3 | 1 | 2 | 6 | Good |
| Ren, H. | 3 | 1 | 2 | 6 | Good |
| Ren, H. | 3 | 1 | 2 | 6 | Good |
| Wang, D. | 3 | 1 | 2 | 6 | Good |
| Wang, J. | 4 | 1 | 1 | 6 | Good |
| Wang, Z. | 3 | 1 | 2 | 6 | Good |
| Yang, X. | 4 | 1 | 1 | 6 | Good |
| Yang, Y. | 3 | 1 | 2 | 6 | Good |
| Yu, Y. | 3 | 1 | 2 | 6 | Good |
| Zhang, B. | 3 | 1 | 2 | 6 | Good |
| Zhao, X. Y. | 3 | 1 | 3 | 7 | Good |
| Yingxia, L. | 3 | 1 | 3 | 7 | Good |
| McMichael, T. M. | 3 | 1 | 2 | 6 | Good |
| Heshui, Sh. | 3 | 1 | 2 | 6 | Good |
| Shi, Q. | 3 | 1 | 2 | 6 | Good |
| The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team | 3 | 1 | 2 | 6 | Good |
| Tang, N. | 4 | 1 | 1 | 6 | Good |
| Wu, Ch. | 3 | 1 | 2 | 6 | Good |
| Zhou, F. | 3 | 1 | 2 | 6 | Good |
| Zhou, Sh. | 3 | 1 | 3 | 7 | Good |
| Wei, X. | 3 | 1 | 2 | 6 | Good |
| Wu, J. | 3 | 1 | 2 | 6 | Good |
| Wu, J. | 3 | 1 | 2 | 6 | Good |
| Pan, L. | 3 | 1 | 2 | 6 | Good |
| An, P. | 2 | 1 | 2 | 5 | Fair |
| Cai, J. | 3 | 1 | 2 | 6 | Good |
| Cao, W. | 3 | 1 | 2 | 6 | Good |
| Chen, J. Y. | 3 | 1 | 2 | 6 | Good |
| Chen, J. | 3 | 1 | 3 | 7 | Good |
| Chen, L. | 3 | 1 | 2 | 6 | Good |
| Chen, N. | 3 | 1 | 2 | 6 | Good |
| Chen, Zh. | 3 | 1 | 2 | 6 | Good |
| Cheng, J. L. | 3 | 1 | 2 | 6 | Good |
| Cheng, Z. | 3 | 1 | 2 | 6 | Good |
| Cui, P. | 3 | 1 | 2 | 6 | Good |
| Fu, B. | 3 | 1 | 2 | 6 | Good |
| Fu, H. | 3 | 1 | 2 | 6 | Good |
| Guan, W. | 3 | 1 | 2 | 6 | Good |
| Henry, B. M. | 3 | 1 | 2 | 6 | Good |
| Huang, C. | 3 | 1 | 2 | 6 | Good |
| Liu, J. | 3 | 1 | 2 | 6 | Good |
| Li, J. | 3 | 1 | 2 | 6 | Good |
| Li, J. | 3 | 1 | 2 | 6 | Good |
| Liu, X. | 3 | 1 | 2 | 6 | Good |
| Li, Y. Y. | 3 | 1 | 2 | 6 | Good |
| Liang, Y. | 3 | 1 | 3 | 7 | Good |
| Liu, K. | 3 | 1 | 3 | 7 | Good |
| Ru, Liu. | 3 | 1 | 3 | 7 | Good |
| Chen, S. | 2 | 1 | 2 | 5 | Fair |
| Colaneri, M. | 3 | 1 | 3 | 7 | Good |
| Xu, Y. | 3 | 1 | 2 | 6 | Good |
| Kang, K. | 3 | 1 | 2 | 6 | Good |
| Liang, T. | 3 | 1 | 2 | 6 | Good |
| Liao, J. | 3 | 1 | 2 | 6 | Good |
| Liu, J. | 3 | 1 | 2 | 6 | Good |
| Liu, K. | 3 | 1 | 2 | 6 | Good |
| Lo, I. L. | 3 | 1 | 2 | 6 | Good |
| Nie, R. | 3 | 1 | 2 | 6 | Good |
| Qiu, Ch. | 3 | 1 | 3 | 7 | Good |
| Qiu, H. | 3 | 1 | 2 | 6 | Good |
| Song, W. | 3 | 1 | 2 | 6 | Good |
| Song, C. Y. | 3 | 1 | 2 | 6 | Good |
| Sun, D. | 3 | 1 | 2 | 6 | Good |
| Wang, K. | 3 | 1 | 2 | 6 | Good |
| Yuan, M. | 3 | 1 | 2 | 6 | Good |
| Zhang, X. | 3 | 1 | 2 | 6 | Good |
| Zhou, F. | 3 | 1 | 2 | 6 | Good |
Newcastle-Ottawa Quality Assessment Form for 215 Cohort Studies (Some of These References Were Used Repeatedly for Different PICO Components; Full References Are Available in Supplementary File)
3.4. Statistical Analysis
MedCalc v.19.0.4 and STATA v.16.0 (Stata Corporation, College Station, TX) were employed to perform the statistical analyses. Comorbidities, signs and symptoms, supportive care, and treatments were measured using the prevalence percentage. I2 statistics and chi-square tests were performed to evaluate the heterogeneity of the studies. The random effect model was used to pool the effect sizes due to inter-study heterogeneity (P-value chi-squared test < 0.1 and I2 > 50%). The following potential moderator variables were employed to conduct subgroup analysis: continent and type of article. No article has been removed from the study by quality assessment. The publication bias was not evaluated because the prevalence as a proportion is always a positive number, and if we saw asymmetry in the funnel design, it is not due to publication bias.
4. Results
4.1. Study Selection
Clinical, diagnostics, treatments, and outcomes in children and adult COVID-19 patients were compared during the first pandemic phase. After searching identified databases, 2022 studies were found, 1,655 were reviewed, and 367 duplicate studies were rejected. After the title and abstract were reviewed, one thousand two hundred thirty-five articles were discarded. By applying the inclusion and exclusion criteria, finally 215 articles left for review (Figure 1). It is worth mentioning that the cited publications were also examined for any relevant research. During the screening process, additional studies were excluded for some other reasons, including lack of numerical values for data analysis (n = 90), unreported confidence intervals (n = 63), and lack of sample size (n = 52). Figure 1 depicts the study selection procedure.
4.2. Results of Quality Assessment
Our findings revealed that 200 studies were of good quality, whereas 15 were of fair quality. No studies were excluded from our study after quality assessment. The results of the quality assessment are presented in Supplementary File.
4.3. Heterogeneity and Synthesis of Results
The I2 index and the chi-square test results showed significant between-study heterogeneity, so results were analyzed by percentages based on the random effect model. The patient’s information was divided into four categories, including signs and symptoms, CT-scan findings, comorbidities, and treatment/outcome. Table 2 summarizes detailed P-values, I2, and confidence intervals. The forest plots of the variables are provided in Supplementary File. Result of meta-analysis and heterogeneity of signs and symptoms, comorbidities, treatments, supportive cares, and CT-scan findings in adults and children with COVID-19 are summarized in Table 2. We also performed subgroup analysis for indicators that had high I2. Its results can be seen in Table 3.
| Group and Subgroup | Population | P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adults | Children | ||||||||
| N | Effect Estimate (Confidence Interval) | I2 | P for Heterogeneity | N | Effect Estimate (Confidence Interval) | I2 | P for Heterogeneity | ||
| Signs and Symptoms | |||||||||
| Fever | 95 | 81.80 (79.06, 84.53) | 95.6% | ≤ 0.001 | 33 | 65.73 (57.30, 74.157) | 98.1% | ≤ 0.001 | ≤ 0.001 a |
| Abdominal pain | 17 | 3.09 (1.39, 4.79) | 94.01% | ≤ 0.001 | 6 | 15.32 (-1.00, 31.64) | 98.37% | ≤ 0.001 | 0.144 |
| Anorexia | 16 | 30.97 (25.55, 36.39) | 90.30% | ≤ 0.001 | 1 | 4.05 (-1.22, 9.32) | NR | NR | ≤ 0.001 a |
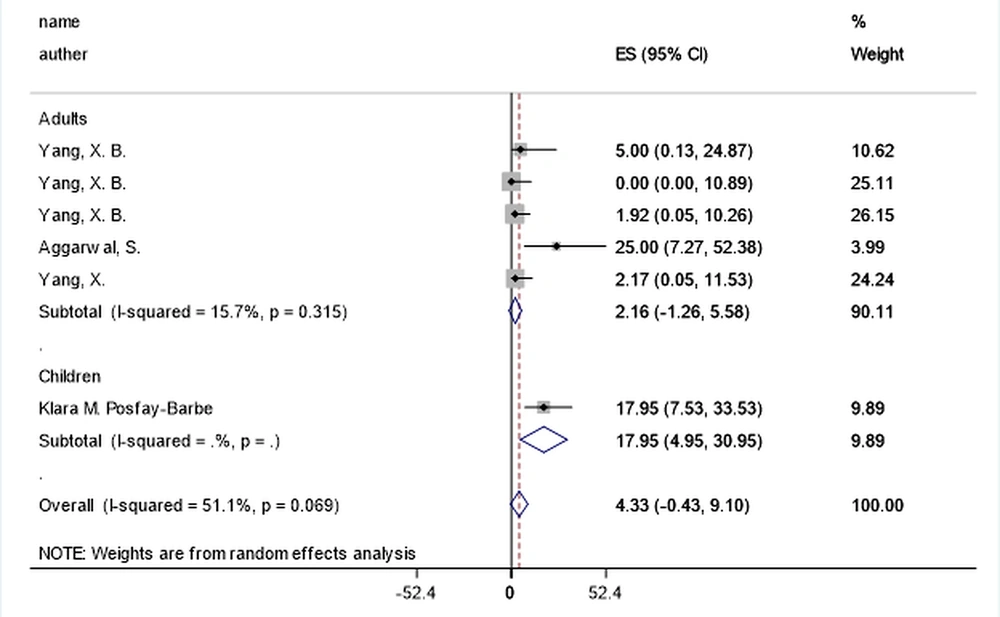
| Arthralgia | 5 | 2.15 (-1.26, 5.57) | 15.7% | 0.315 | 1 | 17.94 (4.94, 30.94) | NR | NR | 0.020 a |
| Bloating | 8 | 1.13 (0.66, 1.60) | 0.00% | 0.913 | 0 | NR | NR | NR | NR |
| Chest pain | 15 | 1.17 (0.39, 1.96) | 10.69% | 0.199 | 4 | 5.27 (-0.44, 10.99) | 83.33% | 0.004 | 0.164 |
| Chest tightness | 19 | 24.57 (18.10, 31.04) | 95.79% | ≤ 0.001 | 0 | NR | NR | NR | NR |
| Chills | 7 | 13.69 (0.24, 27.14) | 99.46% | ≤ 0.001 | 1 | 1.03 (-0.74, 2.82) | NR | NR | 0.067 |
| Cough | 67 | 63.54 (58.48, 68.59) | 96.93% | ≤ 0.001 | 24 | 53.78 (45.14, 62.42) | 94.64% | ≤ 0.001 | 0.056 |
| Delirium | 2 | 2.23 (-3.33, 7.81) | 0.00% | 0.360 | 0 | NR | NR | NR | NR |
| Diarrhea | 59 | 15.80 (12.57, 19.03) | 96.43% | ≤ 0.001 | 17 | 11.43 (5.25, 17.61) | 94.26% | ≤ 0.001 | 0.220 |
| Dizziness | 19 | 3.87 (2.77, 4.97) | 57.33% | 0.003 | 2 | 0.93 (-0.50, 2.37) | 0.00% | 0.473 | 0.001 a |
| Dry cough | 22 | 62.84 (55.58, 70.10) | 95.31% | ≤ 0.001 | 3 | 42.60 (13.04, 72.16) | 71.61% | 0.018 | 0.193 |
| Dyspnea | 47 | 47.64 (41.20, 54.08) | 96.32% | ≤ 0.001 | 8 | 8.81 (0.39, 17.22) | 94.24% | ≤ 0.001 | ≤ 0.001 a |
| Expectoration | 20 | 23.17 (-22.75, 69.10) | 96.63% | ≤ 0.001 | 2 | 37.90 (30.50, 45.30) | 86.07% | 0.007 | 0.535 |
| Fatigue | 58 | 34.39 (30.02, 38.76) | 95.4% | ≤ 0.001 | 5 | 2.63 (0.01, 5.26) | 52.3% | 0.078 | ≤ 0.001 a |
| Gastrointestinal symptoms | 9 | 23.86 (12.34, 35.39) | 98.18% | ≤ 0.001 | 9 | 37.01 (13.69, 60.32) | 98.68% | ≤ 0.001 | 0.322 |
| Headache | 67 | 9.22 (7.52, 10.92) | 91.42% | ≤ 0.001 | 12 | 23.41 (11.68, 35.14) | 96.27% | ≤ 0.001 | 0.019 a |
| Hemoptysis | 10 | 1.79 (0.75, 2.82) | 30.21% | 0.012 | 2 | 11.71 (-6.62, 30.04) | 87.05% | 0.005 | 0.290 |
| Malaise | 14 | 46.14 (37.65, 54.62) | 83.36% | ≤ 0.001 | 2 | 2.67 (0.28, 5.05) | 25.11% | 0.248 | ≤ 0.001 a |
| Muscle ache | 17 | 10.18 (7.85, 12.51) | 80.43% | ≤ 0.001 | 0 | NR | NR | NR | NR |
| Myalgia | 48 | 14.68 (1.36, 28.01) | 93.41% | ≤ 0.001 | 5 | 20.79 (17.13, 24.45) | 97.23% | ≤ 0.001 | 0.386 |
| Nasal congestion or rhinorrhea | 32 | 8.05 (5.96, 10.14) | 88.12% | ≤ 0.001 | 9 | 19.67 (9.47, 29.88) | 87.92% | ≤ 0.001 | 0.029 a |
| Nausea and vomiting | 63 | 6.20 (4.93, 7.46) | 88.40% | ≤ 0.001 | 12 | 12.28 (5.49, 19.07) | 93.53% | ≤ 0.001 | 0.084 |
| Sore throat | 34 | 10.18 (8.28, 12.09) | 81.21% | ≤ 0.001 | 14 | 18.66 (9.40, 27.91) | 96.76% | ≤ 0.001 | 0.079 |
| Sputum | 31 | 26.86 (20.12, 33.61) | 96.65% | ≤ 0.001 | 2 | 9.83 (6.44, 13.22) | 0.00% | 0.704 | ≤ 0.001 a |
| Shortness of breath | 21 | 25.07 (13.47, 36.67) | 99.49% | ≤ 0.001 | 11 | 21.65 (14.81, 28.49) | 86.23% | ≤ 0.001 | 0.619 |
| Computed Tomography (CT)-Scan | |||||||||
| Bilateral pneumonia | 43 | 66.70 (58.62, 74.78) | 99.35% | ≤ 0.001 | 3 | 61.73 (45.62, 77.84) | 30.84% | 0.245 | 0.589 |
| Multiple mottling and ground-glass opacity | 6 | 40.28 (15.79, 64.76) | 98.74% | ≤ 0.001 | 1 | 52.77 (35.72, 69.83) | NR | NR | 0.412 |
| Unilateral pneumonia | 14 | 17.85 (10.83, 24.87) | 97.15% | ≤ 0.001 | 4 | 15.67 (7.82, 23.52) | 3.59% | 0.566 | 0.685 |
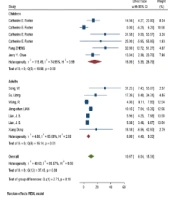
| Normal | 7 | 6.86 (4.39, 9.33) | 65.00% | 0.013 | 6 | 16.09 (5.39, 26.78) | 74.95% | 0.001 | 0.100 |
| Comorbidity | |||||||||
| Asthma | 13 | 8.85 (5.58, 12.12) | 97.57% | ≤ 0.001 | 8 | 15.22 (10.66, 19.79) | 17.94% | 0.486 | 0.026 a |
| Autoimmune disease | 7 | 1.31 (0.53, 2.10) | 0.00% | 0.285 | 0 | NR | NR | NR | NR |
| Cancer | 14 | 5.47 (2.34, 8.60) | 99.70% | ≤ 0.001 | 1 | 10.36 (6.74, 13.98) | NR | NR | 0.045 a |
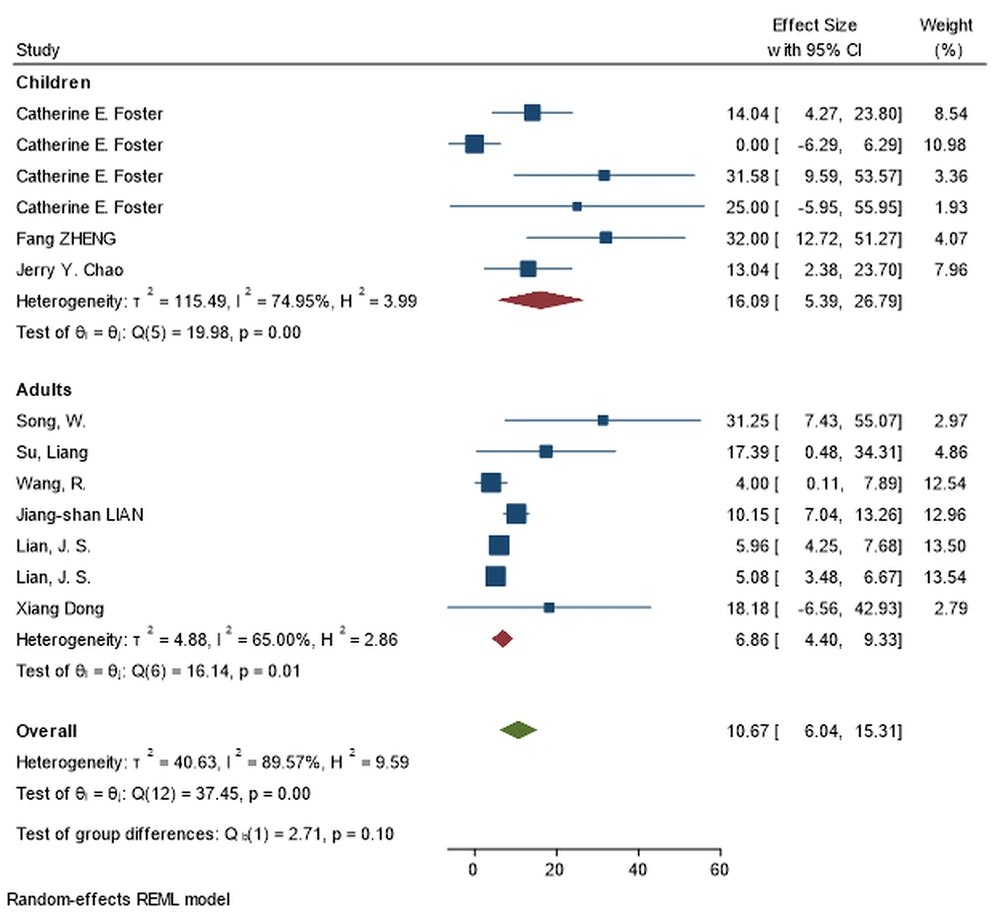
| Cardiovascular disease | 54 | 14.34 (11.24, 17.44) | 99.64% | ≤ 0.001 | 20 | 2.25 (1.14, 3.37) | 89.57% | ≤ 0.001 | ≤ 0.001 a |
| COPD | 71 | 5.59 (4.35, 6.82) | 99.43% | ≤ 0.001 | 2 | 4.92 (3.13, 6.72) | 0.00% | 0.822 | 0.551 |
| Diabetes | 74 | 4.48 (14.94, 19.84) | 99.69% | ≤ 0.001 | 7 | 2.95 (1.41, 4.48) | 0.00 % | 0.893 | ≤ 0.001 a |
| Chronic kidney disease | 36 | 7.46 (4.86, 10.06) | 99.18% | ≤ 0.001 | 6 | 7.27 (-0.28, 14.82) | 98.35% | ≤ 0.001 | 0.962 |
| Chronic liver disease | 31 | 5.61 (2.75, 8.46) | 99.25% | ≤ 0.001 | 1 | 0.93 (-0.32, 2.19) | NR | NR | 0.003 a |
| Obesity | 2 | 37.54 (23.34, 51.74) | 29.02% | 0.235 | 11 | 8.54 (2.84, 14.23) | 92.63% | ≤ 0.001 | ≤ 0.001 a |
| Treatment | |||||||||
| Antibiotic | 13 | 77.86 (66.72, 89.01) | 98.55% | ≤ 0.001 | 8 | 38.01 (23.51, 52.50) | 87.27% | ≤ 0.001 | ≤ 0.001 a |
| ECMO | 11 | 2.74 (0.92, 4.57) | 77.74% | ≤ 0.001 | 2 | 4.46 (1.57, 7.35) | 0.00% | 0.817 | 0.325 |
| Mechanical ventilation | 15 | 32.37 (18.39, 46.35) | 99.31% | ≤ 0.001 | 6 | 7.52 (-0.37, 15.41) | 97.38% | ≤ 0.001 | 0.002 a |
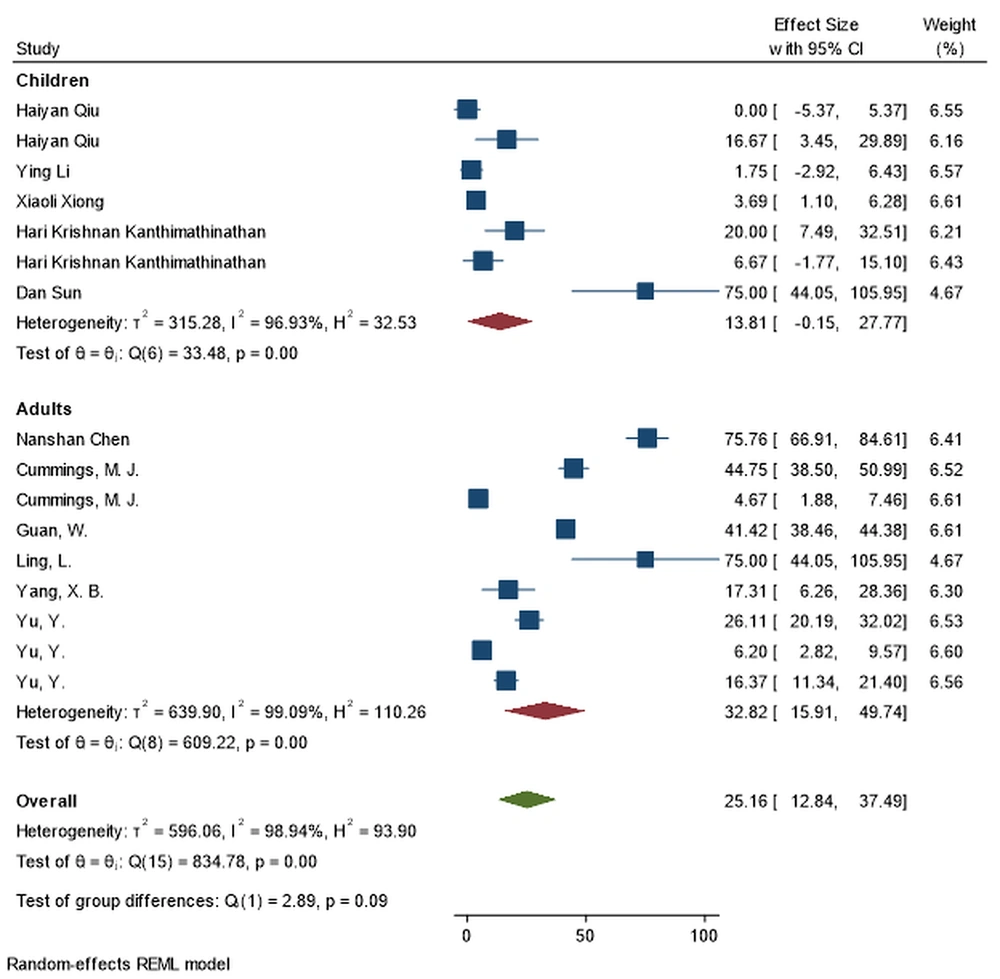
| Oxygen therapy | 9 | 13.81 (-0.14, 27.76) | 99.09% | ≤ 0.001 | 7 | 32.82 (15.90, 49.74) | 96.93% | ≤ 0.001 | 0.089 |
| Hydroxychloroquine | 9 | 66.21 (57.37, 75.06) | 92.25% | ≤ 0.001 | 6 | 29.01 (13.30, 44.72) | 79.82% | 0.001 | ≤ 0.001 a |
| Remdesivir | 7 | 5.41 (1.16, 9.67) | 93.88% | ≤ 0.001 | 4 | 16.86 (5.91, 27.81) | 63.62% | 0.042 | 0.056 |
Result of Meta-Analysis and Heterogeneity of Signs and Symptoms, Comorbidities, Treatments, Supportive Cares, and Computed Tomography (CT)-Scan Findings in Adults and Children with Novel Coronavirus Disease 2019
| Type of Patients | Subgroup | Effect Estimate (Confidence Interval) | I2 % | ||
|---|---|---|---|---|---|
| Adults | Asthma | Type of article | Case Series | 8.30 (-10.83,27.43) | Not reported |
| Cohort | 9.24 (5.32, 13.15) | 98.62 | |||
| Continent | Asia | 0.63 (-0.33, 1.60) | 32.62 | ||
| Europe | 13.98 (11.71, 16.24) | 31.86 | |||
| USA | 11.63 (8.03, 15.22) | 80.30 | |||
| Adults | Cancer | Type of article | Prospective cohort | 9.17 (5.01, 13.34) | 51.55 |
| Retrospective cohort | 4.98 (1.27, 8.69) | 99.79 | |||
| Continent | Asia | 0.60 (0.27, 0.94) | 62.66 | ||
| Europe | 11.27 (1.85, 20.69) | 71.86 | |||
| USA | 11.17 (9.73, 12.61) | 12.65 | |||
| Adults | Cardiovascular disease | Type of article | Prospective cohort | 14.59 (9.29, 19.89) | 99.26 |
| Retrospective cohort | 14.36 (10.61, 18.117) | 99.39 | |||
| Continent | America | 20.30 (13.92, 26.68) | 94.99 | ||
| Asia | 9.06 (6.66, 11.47) | 99.25 | |||
| Europe | 26.91 (15.22, 38.60) | 95.60 | |||
| Children | Cardiovascular disease | Type of article | Retrospective | 2.57 (0.88, 4.26) | 95.83 |
| Prospective cohort | 3.11 (1.27, 4.96) | 37.46 | |||
| Continent | Asia | 3.21 (0.51, 5.92) | 77.38 | ||
| Europe | 3.85 (0.31, 7.38) | 99.12 | |||
| USA | 2.62 (1.33, 3.91) | 5.71 | |||
| Adults | Chronic obstructive pulmonary disease | Type of article | Retrospective | 4.95 (3.73, 6.18) | 97.93 |
| Prospective cohort | 8.30 (5.18, 11.41) | 96.69 | |||
| Continent | Asia | 3.56 (2.72, 4.40) | 95.75 | ||
| Europe | 11.71 (7.55, 15.87) | 88.78 | |||
| USA | 13.51 (8.22, 18.80) | 86.50 | |||
| Adults | Diabetes | Type of article | Retrospective | 17.52 (14.70, 20.35) | 95.42 |
| Prospective cohort | 18.40 (12.93, 23.87) | 99.78 | |||
| Continent | America | 35.94 (32.58, 39.29) | 54.53 | ||
| Asia | 13.37 (11.33, 15.41) | 95.88 | |||
| Europe | 17.56 (12.99, 22.13) | 98.62 | |||
| Adults | Chronic kidney disease | Continent | America | 17.17 (11.03, 23.32) | 94.42 |
| Asia | 2.89 (1.77, 4.02) | 92.68 | |||
| Europe | 11.59 (3.65, 19.54) | 94.87 | |||
| Children | Chronic kidney disease | Continent | Asia | 1.32 (0.15, 2.50) | 50.86 |
| Europe | 17.41 (3.92, 30.90) | 68.90 | |||
| Adults | Chronic liver disease | Type of article | Retrospective | 6.27 (2.86, 9.68) | 72.17 |
| Prospective cohort | 2.98 (1.33, 4.64) | 98.76 | |||
| Continent | America | 1.76 (0.91, 2.61) | 0.00 | ||
| Asia | 5.94 (2.48, 9.40) | 98.72 | |||
| Europe | 3.99 (3.70, 4.29) | NR | |||
| Children | Obesity | Continent | America | 6.98 (1.07, 12.89) | 93.72 |
| Europe | 19.12 (7.50, 30.73) | 0.00 | |||
| Adults | Bilateral pneumonia | Continent | Asia | 65.70 (57.83, 73.57) | 99.19 |
| Europe | 94.00 (90.86, 97.13) | 0.0 | |||
| Adults | Fever | Type of article | Retrospective | 81.88 (78.80, 84.97) | 94.65 |
| Prospective cohort | 92.15 (84.87, 99.43) | 93.29 | |||
| Observational study | 68.15 (57.47, 78.84) | 98.19 | |||
| Continent | America | 79.76 (73.20, 86.32) | 91.46 | ||
| Asia | 82.12 (78.85, 85.38) | 96.47 | |||
| Europe | 39.39 (29.39, 49.39) | Not reported | |||
| Children | Fever | Continent | America | 67.93 (48.19, 87.67) | 96.37 |
| Asia | 59.27 (49.30, 69.24) | 96.44 | |||
| Europe | 81.46 (70.46, 92.46) | 88.85 | |||
| Adults | Abdominal pain | Type of article | Retrospective | 1.47 (0.88, 2.06) | 41.38 |
| Cross-sectional | 9.65 (-5.09, 24.41) | 97.01 | |||
| Children | Abdominal pain | Type of article | Retrospective | 23.00 (0.72, 45.27) | 93.54 |
| Cross-sectional | 15.32 (-1.00, 31.64) | 0.00 | |||
| Continent | Asia | 1.77 (0.22, 3.32) | 0.00 | ||
| Europe | 41.05 (16.31, 65.78) | 83.56 | |||
| Adults | Cough | Type of article | Case series | 46.46 (-9.21, 102.14) | 89.67 |
| Cross-sectional | 58.89 (42.80, 74.98) | 97.81 | |||
| Prospective | 67.97 (64.23, 71.70) | 0.00 | |||
| Retrospective | 67.82 (63.66, 71.98) | 92.91 | |||
| Continent | America | 73.92 (70.42, 77.43) | 55.21 | ||
| Asia | 64.96 (60.34, 69.59) | 94.74 | |||
| Children | Cough | Type of article | Case series | 66.17 (46.33, 86.00) | 53.62 |
| Retrospective | 48.93 (39.19, 58.68) | 95.27 | |||
| Cross-sectional | 42.88 (7.31, 78.45) | 97.52 | |||
| Continent | America | 41.44 (22.8, 60.03) | 94.14 | ||
| Asia | 50.82 (38.60, 63.04) | 96.09 | |||
| Europe | 59.92 (45.51, 74.34) | 84.84 | |||
| Adults | Diarrhea | Type of article | Case series | 9.46 (-3.38, 22.31) | 0.00 |
| Cross-sectional | 17.57 (3.33, 31.82) | 97.21 | |||
| Retrospective | 17.05 (13.40, 20.71) | 96.03 | |||
| Prospective cohort | 6.78 (2.14, 11.42) | 68.51 | |||
| Continent | America | 27.01 (20.80, 33.21) | 88.68 | ||
| Asia | 12.48 (9.24, 15.72) | 96.02 | |||
| Children | Diarrhea | Type of article | Retrospective | 13.09 (5.51, 20.67) | 93.45 |
| Observational study | 4.03 (-0.38, 8.45) | 52.26 | |||
| Continent | America | 8.16 (1.27, 15.04) | 55.76 | ||
| Asia | 3.80 (1.61, 5.99) | 35.02 | |||
| Europe | 27.12 (3.55, 50.68) | 93.50 | |||
| Adults | Dry cough | Type of article | Retrospective | 61.71 (53.94, 69.482) | 95.71 |
| Prospective cohort | 81.56 (75.63, 87.49) | Not reported | |||
| Observational study | 62.31 (33.64, 90.98) | 76.84 | |||
| Adults | Dyspnea | Type of article | Retrospective | 48.26 (41.36, 55.15) | 95.37 |
| Prospective cohort | 57.28 (39.74, 74.83) | 86.56 | |||
| Observational study | 34.34 (8.33, 60.34) | 98.98 | |||
| Continent | America | 67.41 (63.18, 71.65) | 37.63 | ||
| Asia | 41.42 (34.31, 48.54) | 96.44 | |||
| Children | Dyspnea | Type of article | Retrospective | 9.86 (0.59, 19.12) | Not reported |
| Case series | 0.50 (-14.92, 15.92) | 95.36 | |||
| Continent | Asia | 1.40 (-0.08, 2.90) | 3.69 | ||
| Europe | 22.48 (-3.70, 48.68) | 95.33 | |||
| Adults | Fatigue | Type of article | Retrospective | 33.67 (28.23, 39.10) | 96.36 |
| Prospective cohort | 40.95 (34.79, 47.12) | 0.00 | |||
| Observational study | 32.92 (19.34, 46.51) | 96.05 | |||
| Continent | America | 54.25 (49.46, 59.05) | 8.82 | ||
| Asia | 33.19 (28.34, 38.04) | 96.04 | |||
| Children | Gastrointestinal symptoms | Continent | America | 44.74 (-39.68, 129.16) | 98.82 |
| Asia | 19.04 (12.26, 25.83) | 38.84 | |||
| Europe | 49.00 (10.87, 87.12) | 97.53 | |||
| Adults | Headache | Type of article | Observational study | 17.37 (11.57, 23.16) | 84.82 |
| Retrospective | 8.52 (6.78, 10.25) | 90.30 | |||
| Prospective cohort | 5.42 (3.02, 7.81) | 32.03 | |||
| Continent | America | 15.14 (9.89, 20.38) | 89.03 | ||
| Asia | 8.04 (6.41, 9.68) | 89.73 | |||
| Children | Headache | Type of article | Retrospective | 26.52 (13.28, 39.76) | 95.04 |
| Observational study | 4.18 (1.49, 6.88) | 0.00 | |||
| Continent | America | 29.53 (7.80, 51.27) | 91.68 | ||
| Asia | 11.57 (0.11, 23.04) | 94.39 | |||
| Europe | 40.60 (10.69, 70.52) | 88.66 | |||
| Adults | Malaise | Type of article | Retrospective | 47.84 (39.56, 56.11) | 82.25 |
| Case series | 16.66 (-6.49, 39.83) | Not reported | |||
| Continent | America | 58.08 (53.99, 62.17) | 0.00 | ||
| Asia | 29.30 (20.56, 38.03) | 50.80 | |||
| Adults | Myalgia | Type of article | Observational study | 23.90 (13.85, 33.94) | 76.00 |
| Retrospective | 18.98 (14.84, 23.12) | 94.18 | |||
| Prospective cohort | 30.28 (21.73, 38.83) | 80.61 | |||
| Continent | America | 27.95 (25.49, 30.40) | 0.00 | ||
| Asia | 18.50 (14.13, 22.87) | 94.63 | |||
| Adults | Nasal congestion or rhinorrhea | Type of article | Observational study | 5.41 (2.66, 8.16) | 68.66 |
| Retrospective | 8.87 (6.06, 11.68) | 90.43 | |||
| Prospective cohort | 7.39 (4.99, 9.79) | 0.00 | |||
| Continent | America | 14.76 (10.50, 19.03) | 71.12 | ||
| Asia | 4.86 (3.77, 5.96) | 50.50 | |||
| Adults | Nausea and vomiting | Continent | America | 17.25 (15.19, 19.32) | 0.00 |
| Asia | 4.06 (3.46, 4.65) | 45.65 | |||
| Children | Nausea and vomiting | Continent | America | 10.25 (2.11, 18.40) | 65.34 |
| Asia | 7.81 (-0.05, 15.68) | 87.15 | |||
| Europe | 16.40 (-1.17, 33.98) | 94.03 | |||
| Adults | Sore throat | Type of article | Observational study | 17.53 (13.61, 21.45) | 51.63 |
| Retrospective | 9.27 (7.38, 11.17) | 73.29 | |||
| Prospective cohort | 5.83 (3.66, 8.00) | 0.00 | |||
| Continent | America | 7.89 (6.27, 9.51) | 8.07 | ||
| Asia | 11.07 (8.20, 13.93) | 89.32 | |||
| Children | Sore throat | Type of article | Retrospective | 19.07 (8.99, 29.16) | 94.75 |
| Observational study | 18.63 (15.90, 53.17) | 80.69 | |||
| Continent | America | 26.66 (10.50, 42.81) | 86.56 | ||
| Asia | 3.46 (0.69, 6.22) | 47.74 | |||
| Europe | 22.22 (2.75, 47.20) | 87.06 | |||
| Adults | Sputum | Type of article | Retrospective | 27.24 (19.29, 35.19) | 96.91 |
| Prospective cohort | 29.53 (23.76, 35.30) | 0.00 | |||
| Descriptive study | 18.40 (11.86, 48.67) | 98.35 | |||
| Continent | America | 22.76 (19.63, 25.88) | 0.00 | ||
| Asia | 28.27 (19.32, 37.22) | 97.78 | |||
| Adults | Shortness of breath | Type of article | Observational study | 11.84 (5.51, 18.17) | 87.83 |
| Retrospective | 24.33 (8.76, 39.89) | 99.56 | |||
| Prospective cohort | 55.44 (19.28, 91.60) | 99.51 | |||
| Continent | America | 72.34 (69.50, 75.19) | 0.00 | ||
| Asia | 16.99 (7.93, 26.06) | 99.10 | |||
| Children | Shortness of breath | Type of article | Retrospective | 31.24 (17.14, 45.34) | 81.76 |
| Cohort | 16.07 (11.45, 20.69) | 20.16 | |||
| Continent | America | 22.56 (13.13, 31.99) | 86.28 | ||
| Europe | 33.33 (22.73, 43.93) | 0.00 | |||
Result of Subgroup of Signs and Symptoms, Comorbidities, Treatments, Supportive Cares, and Computed Tomography (CT)-Scan Findings in Adults and Children with Novel Coronavirus Disease 2019
4.4. Signs and Symptoms
Fever (65.73 %), cough (53.78 %), expectoration (37.9%), gastrointestinal symptoms (37.01 %), headache (23.41 %), shortness of breath (21.65 %), and myalgia (20.79 %) were the most common symptoms reported in children, according to the pooled estimation analysis (Table 1). Fever (81.8 %) and cough (63.54 %) were the most commonly reported symptoms in adults, similar to children, whereas dyspnea (47.64 %), malaise (46.14 %), fatigue (34.39 %), and anorexia (30.97 %) were more frequently reported in adults.
Fever, anorexia, dizziness, dyspnea, fatigue, malaise, and sputum were markedly higher in adults (P-values = 0.001, 0.001, 0.001, 0.001, and 0.001, respectively), while the arthralgia, headache, and nasal congestion or rhinorrhea were significantly more common among children (P-values = 0.020, 0.019, and 0.029, respectively) (Figure 2). During the beginning phases of the pandemic, the most unusual symptoms were delirium, arthralgia, hemoptysis, and chest pain in adults, and anorexia, malaise, and fatigue in children.
4.5. Computed Tomography (CT)-Scan
Adult patients had a higher rate of abnormal CT-scan findings (including unilateral/bilateral pneumonia and multiple ground-glass opacities) (Figure 3). Children had a greater rate of normal imaging studies.
4.6. Comorbidity
Adults had significantly higher rates of comorbidities such as cardiovascular disease (14.34% vs 2.25%; P-value < 0.001), chronic liver disease (5.61% vs 0.93%; P-value = 0.003), obesity (37.54% vs 8.54%; P-value < 0.001), and diabetes (4.48% vs 2.95%; P-value < 0.001), whereas children had significantly higher rates of asthma (17.94% vs 8.85%; P-value = 0.026) and malignancy (10.36% vs 5.47%; P-value = 0.045) (Figure 4). The most unusual underlying diseases in children were liver disease, cardiovascular disease, diabetes, and kidney disease, respectively. Autoimmune disease is not reported in children.
4.7. Treatment
During initial pandemic phase, hydroxychloroquine (66.21% vs 29.01%; P-value = 0.001) and antibiotics (77.86% vs 38.01%; P-value = 0.001) were used much more frequently in adult patients. Adults are also more likely to require mechanical ventilation (32.37% vs 7.54%; P-value = 0.002) (Figure 4). Extracorporeal membrane oxygenation (ECMO), mechanical ventilation, and remdesivir therapy were infrequently utilized to treat children with COVID-19.
5. Discussion
Despite the appearance of Omicron subvariants, we are nearing the ending of the pandemic, and the current phase appears to be a transition from previous sharp peaks to a less severe surge. So, to better understand SARS-CoV-2 features, it is time to classify pandemic phases based on the different virus strains. In this regard, the current study, which relooked at the COVID-19 pandemic in its initial stages, can be considered pioneering. Early in the pandemic, the clinical characteristics of pediatric patients with SARS-CoV-2 infection were not well recognized (9). The original SARS-CoV-2 infection primarily affects adults, whereas newer variations of concern (VOC) variably affect children. In critically ill adult patients, the beta variant did not vary from the alpha variant in terms of patient characteristics, management, or outcomes (10). In the United States, the first occurrence of multisystem inflammatory syndrome in children (MIS-C) started shortly after the beta variant peak (11). Furthermore, the alpha variant has been associated with an increase in COVID-19 cases and hospitalizations among young people (12). In children under the age of five, the incidence rate of SARS-CoV-2 infection with the Omicron variant was six to eight times that of the delta variant, but overall severe clinical consequences were less common than with the delta variant (13). However, there has been some concern about the risk of cardiac arrest associated with the Omicron variant’s rapid-onset upper airway obstruction (14).
As earlier noted, new VOC-related clinical manifestations changed quickly, particularly in children infected with COVID-19; however, based on our findings in the early pandemic phase, fever and cough were the most common symptoms in both adults and children. Therefore, the patient’s age is one of the main determinants of different disease phenotypes (15-17). The preponderance of arthralgia and headache in children was one of the key findings in our study, whereas other studies observed a higher proportion of headaches in adults (18, 19). Furthermore, nausea and vomiting, chest pain, gastrointestinal symptoms, and gastric pain are all insignificantly higher in children, according to our findings. Adult patients had considerably higher rates of fever, fatigue, and dyspnea. Moreover, no cases of chest tightness, confusion, or myalgia were found in children. Although there was no statistical difference in CT-scan findings, children had more normal imaging studies. Our findings are similar to that of another systematic review on children, which found that more than a third of COVID-19 patients had a normal chest CT scan (20). Small subpleural nodular ground-glass opacity is the most common CT-scan abnormality in children infected with the original strain (21). Patchy shadows, ground-glass opacities, consolidation, partial air bronchogram signs, nodules, and halo signs were the most commonly reported pulmonary manifestations in a systematic review conducted before the resurgence of the alpha, delta, gamma, and Omicron strains, while pleural effusion and paving pattern were rare (22). Besides, the delta variant showed fewer abnormalities than the original strain, mainly found in the lower lungs on both sides confined in a single lobe (unlike the initial strain, which was distributed over multiple lobes) (21). Unilateral/bilateral pneumonia, multiple mottling, and ground-glass opacities were detected in children and adults infected with the original SARS-CoV-2 strain without statistically significant differences, according to our data acquired during the initial pandemic phase.
Adult COVID-19 cases had significantly more comorbidities, such as cardiovascular disease, obesity, and diabetes; however, our findings show that the risk of malignancy is significantly higher in infected children. Following the emergence of the Omicron variant, the incidence of pediatric cases has considerably increased. Even though children’s symptoms are milder than adults’, severe disease can still occur, especially in children with comorbidities (23).
Poor outcomes were connected to heart failure, acute respiratory distress syndrome (ARDS), and renal failure. Compared to non-severe cases, individuals with severe COVID-19 more often need mechanical ventilation and renal replacement therapy. Prehospital comorbidities are an important factor in children as well (24). according to evidence from Middle East respiratory syndrome (MERS) or the flu, patients who have been given corticosteroids have longer viral replication, need mechanical ventilation, and have a higher death rate (25-28). However, systemic corticosteroids may improve all-cause mortality in critically ill patients with COVID-19, according to the results of a meta-analysis of clinical studies (29). Antibiotics are prescribed to most COVID-19 patients, even though the estimated prevalence of bacterial co-infection is much lower (30). In patients with COVID-19, the usage of unnecessary antibiotics is likely to be considerable. In addition, we found that adults used much more antibiotics than children. Nonetheless, antibiotics were given to around 40% of the children studied.
This meta-analysis has some advantages. The most remarkable advantage of our study may be the investigation of COVID-19 characteristics in adult and pediatric patients during the early phases of the pandemic, which is carried entirely during the wild-type SARS-CoV-2 (Wuhan’s strain) circulation in the first six months of the pandemic. Given that the beta, alpha, delta, and gamma strains were discovered in May, September, October, and November of 2020, respectively, the current study mainly analyzes clinical, and radiological findings and clinical outcomes of the disease during the COVID-19 initial phase induced by Wuhan strain. Second, we collected our data from more than 25,000 articles based on five different databases, increasing the comprehensiveness of this review. Third, we used almost all data about pediatric patients in our systematic review; however, articles about pediatric patients were published later. Fourth, our results were analyzed by two different software, and three experts checked quantitatively and qualitatively. Finally, the authors recommend that future studies focus on the various phases of the pandemic when performing similar studies. There are some limitations to our study. For example, the results could have been influenced by different research locations, methodology, and outcome measures. Another drawback was the inclusion of different types of research, which may inadvertently influence the conclusions based on the strengths and limitations of each study.
6. Conclusions
Although children were afflicted less than adults in the early stages of the pandemic and had lower mortality, clinical and radiological findings, as well as prognostic factors, did not differ significantly between adults and children. However, with the introduction of novel variants, clinical signs and symptoms, complications, and outcomes have all changed significantly in children.