1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a variant of coronaviruses discovered in late 2019 (1). Children generally account for a small proportion of disease instances and usually present with mild forms of the disease (2). Nonetheless, on April 27, 2020, the Pediatric Intensive Care Society warned about the increasing number of pediatrics with the severe inflammatory syndrome and Kawasaki disease-like features, who mostly were SARS-CoV-2-positive or had COVID-19 exposure (3, 4). Consequently, in mid-May 2020, the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) published sets of case definition criteria for the so-called multisystem inflammatory syndrome in children (MIS-C) for disease surveillance. Causing hyperinflammatory syndrome with multisystem involvements (above two organs), MIS-C is considered the severe form of COVID-19 in children and an indication for hospitalization (5, 6).
Pneumothorax is divided into spontaneous and traumatic/Iatrogenic entities. Spontaneous pneumothorax can occur in people without (primary type) or with (secondary type) a pre-existing chronic lung disease or positive history of trauma (7). Although several cases of spontaneous pneumothorax are reported in COVID-19, mostly elderly male patients with underlying conditions (8, 9); it is even rarer in pediatric COVID-19 patients (10) presented in a few case reports (11-14). Moreover, bilateral spontaneous pneumothorax is reported much less than unilateral cases (11, 14, 15). Herein, we report the case of a five-year-old boy with MIS-C associated with COVID-19 who developed bilateral spontaneous pneumothorax during hospital admission.
2. Case Presentation
2.1. Medical History
A previously healthy Afghan boy, five-year-old, 106-cm tall (25th percentile), and 15 kg weight (third percentile), was referred to a pediatric emergency department with the chief complaints of abdominal pain and fever. The patient was healthy until five days before admission, when he initially developed a fever. Two days later, generalized rashes appeared on his abdomen that extended to his extremities. Consequently, he developed diarrhea, abdominal pain, and abdominal distention. His caregivers did not suspect that he might have had close contact with a positive COVID-19 patient during the last month. He had neither underlying medical conditions nor previous hospital admissions and surgeries. Also, he did not use any medications, had no history of allergic reactions, and was fully vaccinated against COVID-19.
2.2. Presenting Symptoms and Clinical Examination
On physical examination, his vital signs were: a body temperature of 39°C (orally), heart rate of 145 beats/min, respiratory rate of 40 breaths/min, blood pressure of 90/50 mmHg, and arterial oxygen saturation of 90% (breathing the ambient air). On arrival, he presented with red, cracked lips, an erythematous throat, and generalized maculopapular rashes on his abdomen, chest, and extremities. He had suprasternal and subcostal retraction and nasal flaring. Also, wheezing was auscultated. Moreover, he had abdominal distention with mild tenderness but without hepatosplenomegaly. The rectal sphincter tone was normal, and he passed a small amount of yellowish watery stool after rectal examination. No lymphadenopathy was detected.
2.3. Paraclinical Findings
Blood tests showed lymphocytopenia, anemia, thrombocytopenia, increased levels of D-dimer, troponin, interleukin-6, prohormone of brain natriuretic peptide, prothrombin time, partial thromboplastin time, and decreased levels of potassium, magnesium, calcium, and albumin (Table 1).
| Test | Result | Reference Range |
|---|---|---|
| Anti-SARS-CoV-2 serologic immunoassays | ≥ 1.1 | |
| Immunoglobulin M (IgM) | 1.5 | |
| Immunoglobulin G (IgG) | 10.5 | |
| Hemoglobin (g/dL) | 8.1 | 11.5 - 14 |
| White blood cell count (cells/µL) | 5,500 | 5,000 - 17,000 |
| Absolute lymphocyte count (cells/µL) | 935 | 1,500 - 9,500 |
| Platelet count (×1000 cells/µL) | 100 | 150 - 400 |
| Sodium (mEq/L) | 135 | 136 - 145 |
| Potassium (mEq/L) | 2.7 | 3.5 - 5.5 |
| Magnesium (mg/dL) | 1.2 | 1.6 - 2.5 |
| Calcium (mg/dL) | 6.8 | 8.6 - 10.3 |
| Creatinine (mg/dL) | 0.66 | 0.8 - 1.3 |
| Lactate dehydrogenase (U/L) | 1,142 | < 480 |
| C-reactive protein (mg/L) | 150 | 0.6 - 7.9 |
| Erythrocyte sedimentation rate (mm/h) | 88 | 3 - 13 |
| D-dimer (ng/mL) | > 10,000 | < 500 |
| Troponin (ng/L) | 107 | < 19 |
| Prohormone of brain natriuretic peptide (pg/mL) | 82 | < 70 |
| Aspartate aminotransferase (U/L) | 70 | < 37 |
| Alanine aminotransferase (U/L) | 65 | < 41 |
| Albumin (g/dL) | 2.7 | 3.8 - 4.2 |
| Prothrombin time (s) | 17.7 | 12 - 14 |
| International normalized ratio | 1.28 | 0.9 - 1 |
| Partial thromboplastin time (s) | 69 | 25 - 35 |
| Fibrinogen (mg/dL) | 400 | 200 - 400 |
| Ferritin (ng/mL) | 575 | 7 - 140 |
| Interleukin-6 (pg/mL) | 31 | < 7 |
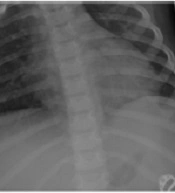
On admission, a chest roentgenogram (CXR) showed mild diffuse pulmonary infiltration and ground-glass opacities accompanied by faint air bronchograms in both lung fields (Figure 1A). In addition, spiral chest computed tomography (CT) showed diffuse subpleural ground-glass densities associated with multiple subpleural and posterior nodules in lung fields, suggestive of SARS-CoV-2 infection. A mild left-sided pleural effusion was also noted (Figure 1D-G). Abdominopelvic sonography showed a minimal amount of free fluid in the abdominal cavity. Generalized bowel loop dilatation was noted in abdominal CT. Transthoracic echocardiography was normal.
[Top row] Sequential plain posteroanterior (PA) chest X-ray (CXR) images taken on different days of hospital stay. (A) On admission, PA CXR shows mild diffuse pulmonary infiltration and ground-glass opacities accompanied by faint air bronchograms in both lung fields; (B) PA CXR on the fifth day of admission reveals mild left-sided, and moderate right-sided parenchymal lung collapses associated with bilateral air bronchograms, and visible pleural lines consistent with moderate right-sided pneumothorax and mild left-sided pneumothorax; (C) AP CXR after bilateral chest tube insertion on the same day. Chest tubes are properly placed in pleural spaces. Well-expanded lungs with no residual pneumothorax are noted. [Middle and down rows] Spiral chest CT scan cuts in the axial plane, lung window, at different levels of lungs on the first day of admission. (D & E) Below the level of the carina, diffuse subpleural ground-glass densities associated with multiple peripherally located (dominantly subpleural and posterior) pulmonary nodules with peripheral halos are seen in both lung fields. Also, several bilateral reactive hilar lymph nodes are noted on the mediastinal window; (F) At the level of the left ventricle, multiple areas of ground-glass densities associated with peripherally and centrally located pulmonary nodules demonstrating peripheral halos are observed in posterior aspects of the lung fields. Mild left-sided pleural effusion is also noted; (G) At the diaphragm level, multiple areas of subpleural ground-glass densities associated with few pulmonary nodules are seen in the bases of the lungs, prominent on the left. Mild left-sided pleural effusion is demonstrated.
Reverse-transcription polymerase chain reaction (RT-PCR) against SARS-CoV-2 was performed twice via nasopharyngeal swabs, which were negative. However, serological immunoassay showed positive results for immunoglobulin G (IgG) and immunoglobulin M (IgM) against SARS-CoV-2 antigens (Table 1).
2.4. Therapeutic Focus
The patient was given oxygen with a non-rebreather mask. After consultation with pediatric surgeons, they recommended supportive therapy for his abdominal distention; therefore, nasogastric and rectal tubes were inserted in addition to administering intravenous broad-spectrum antibiotics. During the first hours of hospitalization, he had no urine output. Shock management was performed, along with intravenous (IV) hydration. The fever subsided a few hours after administering 2 g/kg of IV immunoglobulin (IVIG) and 2 mg/kg/day of methylprednisolone. Duolin® inhaler (ipratropium bromide 500 µg + levosalbutamol 1.25 mg per each 2.5-mL of respule) (0.15 mg/kg) was also administered because of the persistent wheezing.
On the second day of the hospital stay, he developed generalized edema. Hence, IV fluids were restricted, and 10 g/day of albumin and 0.5 mg/kg/day of furosemide were infused. After three days, his condition improved; his vital signs turned stable, abdominal distention subsided, he no longer had tachypnea or respiratory distress, and his edema decreased. A liquid diet was initiated, which was well tolerated.
However, on the fifth day of the hospital stay, he developed dyspnea, and his condition worsened. CXR was carried out and revealed mild left-sided and moderate right-sided parenchymal lung collapses associated with bilateral air bronchograms and visible pleural lines consistent with moderate right-sided and mild left-sided pneumothorax (Figure 1B). Hence, bilateral chest tubes were inserted, and his condition was returned to stable (Figure 1C).
Five days later, the chest tubes were removed, and he was discharged one week after. Follow-up echocardiography was performed two weeks later, which showed normal cardiac function and coronary artery dimensions. Two months later, he was in good health on the second appointment.
3. Discussion
Spontaneous pneumothorax is rarely reported in pediatric COVID-19 patients (8, 10). A systematic review of 37 articles on CT features in pediatric COVID-19 patients found only two cases of pneumothorax and one case of bullae, none of which was bilateral (10). Hashemi et al. (12) described a two-year-old boy with hyper IgM syndrome and COVID-19 infection who developed unilateral spontaneous pneumothorax during the hospital stay. Also, Montgomery and Finck (13) described a 17-year-old boy with COVID-19 infection who presented with unilateral hemopneumothorax, which was exacerbated during the hospital stay. Furthermore, Dixit et al. (14) reported a three-month-old boy with COVID-19 infection who presented with a massive bilateral pneumomediastinum and subcutaneous emphysema. Our case is the second report of MIS-C associated with COVID-19 who had multisystem involvement, including cardiovascular, respiratory, gastrointestinal, hematological, and dermatological systems, and developed bilateral primary spontaneous pneumothorax. To the best of our knowledge, there is only one report about MIS-C complicated with spontaneous bilateral pneumothorax (11). Laaribi et al. (11) described two boys with COVID-19 (nine-month-old and 18-month-old) who developed bilateral spontaneous pneumothorax. They did not mention MIS-C associated with COVID-19 in their two cases; however, by definition (6), MIS-C is evident. In addition, contrary to our case, pneumothorax was developed before admission in those two cases.
We could not find the definite cause of bilateral spontaneous pneumothorax in our patient. Barotrauma associated with positive pressure mechanical ventilation can lead to pneumothorax (16). In Zantah et al. analysis of 902 COVID-19 patients, all six patients who developed pneumothorax had a unilateral pathology, four of whom were associated with mechanical ventilation (17). Our case did not receive any ventilation supports before pneumothorax diagnosis, reflecting that barotrauma could not be the cause of pneumothorax. Interestingly, in all similar cases (11-14), barotrauma was also ruled out. One plausible explanation might be excessive coughing, which is common in COVID-19 patients. It can increase intra-thoracic and alveolar pressure. In addition, COVID-19 pneumonia can cause alveolar damage directly (18). Furthermore, hyperinflammatory status is linked to more severe lung injuries (i.e., adults with severe COVID-19 who developed pneumothorax) (19, 20). Interestingly, our case had multisystem involvements and laboratory evidence of hyperinflammatory status. Overall, bilateral primary spontaneous pneumothorax in our patient might be attributed to the inflammatory reactions secondary to COVID-19.
3.1. Conclusions
In conclusion, children with MIS-C associated with COVID-19 may develop spontaneous pneumothorax during the hospital stay, without barotrauma or previous underlying medical conditions. Owing to the clinical picture overlapping with MIS-C associated with COVID-19, the timely diagnosis of pneumothorax may be challenging in such patients.
![[Top row] Sequential plain posteroanterior (PA) chest X-ray (CXR) images taken on different days of hospital stay. (A) On admission, PA CXR shows mild diffuse pulmonary infiltration and ground-glass opacities accompanied by faint air bronchograms in both lung fields; (B) PA CXR on the fifth day of admission reveals mild left-sided, and moderate right-sided parenchymal lung collapses associated with bilateral air bronchograms, and visible pleural lines consistent with moderate right-sided pneumothorax and mild left-sided pneumothorax; (C) AP CXR after bilateral chest tube insertion on the same day. Chest tubes are properly placed in pleural spaces. Well-expanded lungs with no residual pneumothorax are noted. [Middle and down rows] Spiral chest CT scan cuts in the axial plane, lung window, at different levels of lungs on the first day of admission. (D & E) Below the level of the carina, diffuse subpleural ground-glass densities associated with multiple peripherally located (dominantly subpleural and posterior) pulmonary nodules with peripheral halos are seen in both lung fields. Also, several bilateral reactive hilar lymph nodes are noted on the mediastinal window; (F) At the level of the left ventricle, multiple areas of ground-glass densities associated with peripherally and centrally located pulmonary nodules demonstrating peripheral halos are observed in posterior aspects of the lung fields. Mild left-sided pleural effusion is also noted; (G) At the diaphragm level, multiple areas of subpleural ground-glass densities associated with few pulmonary nodules are seen in the bases of the lungs, prominent on the left. Mild left-sided pleural effusion is demonstrated. [Top row] Sequential plain posteroanterior (PA) chest X-ray (CXR) images taken on different days of hospital stay. (A) On admission, PA CXR shows mild diffuse pulmonary infiltration and ground-glass opacities accompanied by faint air bronchograms in both lung fields; (B) PA CXR on the fifth day of admission reveals mild left-sided, and moderate right-sided parenchymal lung collapses associated with bilateral air bronchograms, and visible pleural lines consistent with moderate right-sided pneumothorax and mild left-sided pneumothorax; (C) AP CXR after bilateral chest tube insertion on the same day. Chest tubes are properly placed in pleural spaces. Well-expanded lungs with no residual pneumothorax are noted. [Middle and down rows] Spiral chest CT scan cuts in the axial plane, lung window, at different levels of lungs on the first day of admission. (D & E) Below the level of the carina, diffuse subpleural ground-glass densities associated with multiple peripherally located (dominantly subpleural and posterior) pulmonary nodules with peripheral halos are seen in both lung fields. Also, several bilateral reactive hilar lymph nodes are noted on the mediastinal window; (F) At the level of the left ventricle, multiple areas of ground-glass densities associated with peripherally and centrally located pulmonary nodules demonstrating peripheral halos are observed in posterior aspects of the lung fields. Mild left-sided pleural effusion is also noted; (G) At the diaphragm level, multiple areas of subpleural ground-glass densities associated with few pulmonary nodules are seen in the bases of the lungs, prominent on the left. Mild left-sided pleural effusion is demonstrated.](https://services.brieflands.com/cdn/serve/3170b/88ce49f6d89ff45a965520185393bf70fe18de7f/apid-128964-g001-F1-preview.webp)