1. Background
Toxoplasma gondii is a pathogenic protozoan that causes toxoplasmosis and spreads worldwide. The infection is transmitted via ingestion of drinking water, food, or soil contaminated with the oocysts in the cat’s feces and consumption of raw meat (1). Other transmission routes are organ transplantation and congenital transmission of toxoplasmosis (2-4). The infection has been reported only based on regional studies. Therefore, the prevalence of toxoplasmosis is different and depends on the geographical and socio-economic situation, climatic elements, and food culture in a region (5, 6).
It has been reported that one-third of the global population is infected with T. gondii. The seroprevalence rate of toxoplasmosis among the Iranian population is 39.3% (7). Overall, the seroprevalence rate of T. gondii IgG was reported at 56.3%, and the rate of IgM has been reported to be 3.7% among Iranian adolescents aged 10 - 18 years (8). Usually, the infections are asymptomatic in immunocompetent individuals, and cervical lymphadenopathy or ocular disease happens in up to 10% of infected patients (9); however, immunocompromised patients are symptomatic, and they may develop myocarditis, encephalitis, or pneumonitis (10).
The street children have a low level of hygiene and lack access to the health system. These children are exposed to infectious diseases due to inadequate personal and environmental hygiene, so worldwide consideration is needed to legalize working children (11). To date, no study has been done on the prevalence of toxoplasmosis in children, especially among working children in Tehran.
2. Objectives
This study aimed to investigate the prevalence of toxoplasmosis by serological and molecular methods in working children and the control group in Tehran.
3. Methods
3.1. Sample Collection
The Sobh-e Rooyesh is a school that is associated with working children. It was founded in 2014 in Tehran and is the first school for working children in Iran. The students are educated in different grades and work outside school hours. The children evaluated in this study returned to their place of residence or their relatives at night, and some lived with their father or mother. This study was conducted in 2021. Sampling was carried out by accidental method according to the formula:
where P is the seroprevalence of toxoplasmosis (22%), d is 0.038 marginal error, and standard score (Z) is 95% confidence interval. No data about the seroprevalence of toxoplasmosis in working children was available; however, the seroprevalence of toxoplasmosis among children was reported between 17.7% - 22.1% in different cities in Iran, including Tehran, sari, and Bushehr (12-15). The sample size was calculated at 458, and finally, the sample size used in the study was 460.
A total of 460 children between the ages of seven and 14 years, including 278 working children affiliated with Sobh-e Rooyesh school and 182 age-matched control children were sampled from different laboratories in Tehran. Commercial enzyme-linked immunosorbent assay (ELISA) kits were used to evaluate IgM and IgG antibodies against T. gondii. Blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and used to isolate peripheral blood mononuclear cells (PBMCs) by gradient centrifugation method. Real-time polymerase chain reaction (PCR) was performed using primer B1 on PBMC samples in children’s blood to determine the status of Toxoplasma infection.
3.2. Enzyme-linked Immunosorbent Assay
The present study evaluated IgG and IgM antibodies against T. gondii among children using an ELISA kit (EUROIMMUN, Germany). For IgG antibodies, titers were considered positive if they were > 11 IU/mL, borderline if they were 8 - 11 IU/mL, and negative if they were < 8 IU/mL. For IgM antibodies, titers were regarded as positive if they were > 1.1 IU/mL, borderline range between 0.8 - 1.1 IU/mL, and negative if they were < 0.8 IU/mL.
3.3. Real-time Polymerase Chain Reaction
The genomic DNA from PBMC samples was extracted using the QIAamp DNA extraction kit (Qiagen, Hilden, Germany). The real-time PCR test was carried out using the T. gondii B1 gene (B22, B23) that amplified a 115 bp fragment. The reaction was done using the Master SYBR Green I (Roche Molecular Biochemicals). Each primer was added at the concentrations of 0.5 μM for B1 in a volume of 20 μL. All primer sequences and PCR conditions used in this study were derived from a previous study (16).
3.4. Statistical Analysis
The analysis was performed by SPSS version 18 (Chicago, IL, USA), and chi-square and a P-value of ≤ 0.05 was considered statistically significant.
4. Results
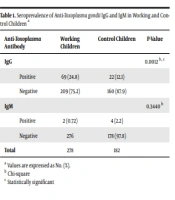
Seroprevalence of IgG and IgM antibodies against T. gondii among working children and the control group is summarized in Table 1. The present study results indicated that the frequency of IgG antibodies between working children (24.8%) and the control group (12.1%) was statistically significant (P = 0.0012). However, there was no significant difference in IgM seropositivity between the two groups (P = 0.3440, Table 1).
a Values are expressed as No. (%).
b Chi-square
c Statistically significant
The number of IgG seropositive cases for T. gondii with a titer above 200 IU/mL was 32 in working children. In contrast, only five subjects were IgG seropositive above 200 IU/mL for T. gondii among children in the control group. The results also showed 19 cases with a titer of 100 - 200 IU/mL in working children, while one case with this titer was found in the control group. The number of cases with a titer of 10 - 100 IU/mL was 18 in working children and 16 in the control group. The statistical analysis showed a significant difference between the two groups regarding high IgG antibody titer against T. gondii (P = 0.00332) (Table 2). The mean IgG titer was 160 ± 86.39 IU/mL and 69.36 ± 88 IU/mL for working children and the control group, respectively (P < 0.0001); however, the mean IgM titer was 4.65 ± 3.04 IU/mL and 3.85 ± 4 IU/mL for working children and control group, respectively (P = 0.8187).
a Values are expressed as No. (%).
b Chi-square
c Statistically significant
In the present study, the relationship between the seroprevalence of toxoplasmosis and gender was not statistically significant in working children and the control group (P = 0.2333). In contrast, among the working children group, the seroprevalence of toxoplasmosis in the age group of 11 - 14 years was higher than in the age group of 7 - 10 years, and the difference between the two groups was statistically significant (P = 0.04, Table 3). Some of the hygienic habits of the children are summarized in Table 3. Unwashed hands with soaps before eating and contact with soil and cats were some of the hygiene habits of these children. Among these factors, only unwashed hands before eating (P = 0.0115) and contact with soil were statistically associated with toxoplasmosis rate (P = 0.0005).
| Variables | Working Children | P-Value | Control Children | P-Value | ||
|---|---|---|---|---|---|---|
| No. Tested | Positive | No. Tested | No. Positive | |||
| Gender | 0.3643 b | 0.2333 b | ||||
| Boys | 146 | 40 (27.4) | 90 | 14 (15.5) | ||
| Girls | 132 | 29 (21.9) | 92 | 8 (8.7) | ||
| Age group (y) | 0.04 b, c | 0.6293 b | ||||
| 7-10 | 96 | 16 (16.7) | 84 | 10 (11.9) | ||
| 11-14 | 182 | 50 (27.5) | 76 | 12 (15.7) | ||
| Contact with soil | 0.0005 b, c | 0.4282 b | ||||
| Yes | 153 | 51 (33.3) | 93 | 9 (9.7) | ||
| No | 125 | 18 (14.4) | 89 | 13 (14.6) | ||
| Eating with unwashed hands | 0.0115 b, c | 0.7729 b | ||||
| Yes | 195 | 55 (28.2) | 33 | 3 (9.1) | ||
| No | 83 | 11 (13.2) | 149 | 19 (12.7) | ||
| Contact with cat | 0.0879 b | 0.1992 b | ||||
| Yes | 15 | 7 (46.7) | 22 | 5 (22.7) | ||
| No | 263 | 62 (23.6) | 160 | 17 (10.6) | ||
| Total | 278 | 69 | 182 | 22 | ||
a Values are expressed as No. (%).
b Chi-square
c Statistically significant
4.1. Real-time Polymerase Chain Reaction Results
Real-time PCR results indicated two (0.7%) positive cases among working children, and these two cases had anti-T. gondii IgM positive results and IgG antibody with a titer higher than 200 IU/mL; however, real-time PCR was positive in three (1.65%) samples in the control group. These three cases had anti-T. gondii IgM. In one case, the antibody IgM against T. gondii was positive, but the real-time PCR result was negative. Finally, real-time PCR indicated five (1.1%) positive results among 460 children in our study. There was no significant difference in real-time PCR results between working children and the control group (P = 0.35) and real-time PCR and IgM seropositive results between the two groups (P = 0.81, Table 4). For three months, we followed up on four children with anti-T. gondii IgM-positive results in the control group for T. gondii IgM antibody and genomic DNA by ELISA and real-time PCR assays, respectively. The results indicated that IgM remained positive with lower titers in two control group children; however, PCR results were negative.
| Groups | Serological Tests Enzyme-linked Immunosorbent Assay | Real-time Polymerase Chain Reaction | P-Value | |
|---|---|---|---|---|
| IgM+ | IgG+ | |||
| Working children (278) | 2 (0.7) | 69 (24.8) | 2 (0.7) | 0.81 b |
| Control children (182) | 4 (2.2) | 22 (12.1) | 3 (1.6) | |
| Total (460) | 6 (1.3) | 91 (19.8) | 5 (1.1) | |
a Values are expressed as No. (%).
b Chi-square between IgM and real-time polymerase chain reaction
5. Discussion
In the current survey, the seroprevalence of T. gondii IgG antibody was 24.8% in working children, and 0.7% of the positive samples were IgM-seropositive for T. gondii. It should be noted that these two cases had IgG antibodies with a titer above 200 IU/mL. In the control group, 12.1% had anti-T. gondii IgG antibody and seroprevalence of T. gondii IgM was 2.2%. The seroprevalence of T. gondii has been reported at 17.7% by ELISA and IFA methods in high school girls in the Robat-Karim area of Tehran (12). The seroprevalence of T. gondii was 22% using ELISA in schoolchildren in Sari, Iran, and the age group of more than 11 years had a higher seroprevalence than 7 - 10 years (13).
In a study conducted in Bushehr on high school girls aged 15 - 18 years, 22.1% and 1.4% were seropositive for IgG and IgM antibodies against T. gondii, respectively, and approximately 88% of girls were seronegative for T. gondii antibody (14). In a study conducted in Isfahan on high school girls aged 14 - 19, the seroprevalence of T. gondii was evaluated using ELISA and IFA methods and the total anti-T. gondii seropositive rate was 18.4%, which increased with age (15). A study was conducted in Fasa city of the Fars province on 947 high school girls aged 14 - 19 to evaluate the seroepidemiology of T. gondii by ELISA method and the overall anti-T. gondii seropositivity rate was 10.1% (17). In general, the highest infection rates have been reported in Northern provinces, including Mazandaran and Guilan; however, the infection rate is low in south of Iran (13, 17). These differences can be related to humid and temperate air conditions in the north of Iran and dry and warm conditions in the south of Iran. The presence of cats is another factor in these differences (16).
However, seroepidemiological studies of T. gondii have demonstrated a low prevalence in children in some countries, including Pakistan (17.4%) (18), China (16%) (19), Romania (16.6%) (20), Ireland (12.8%) (21) and Korea (12.6%) (22), the overall prevalence of 12.5% for the seven districts in the United Arab Emirates, including 3.5% for Dubai and 34.6% for Sharjah (23). It may be due to hygienic habits, climate conditions, and food culture for consumption of vegetables and meat and contact with cats. In the present research, working children exposed to contaminated soil, water, or food without good hygiene levels had a higher seroprevalence of toxoplasmosis than children in the control group who were less exposed to soil, contaminated water, or food and showed a better level of health. Therefore, the numbers of positive cases (24.8%) in working children were more than in the control children (12.1%), and the differences were statistically significant. Also, the number of seropositive cases of IgG antibody against T. gondii with a titer above 200 IU/mL was 32 cases, with only five cases in the children of the control group. Titers of 100 - 200 IU/mL were found in 19 cases in the working children and one case in the control group, and the difference was significant.
This survey showed that the seroprevalence of toxoplasmosis is higher in frequency and antibody titer among working children. The mean IgG titer in working children was more than in the control group, and the result was statistically significant. More contact with the soil and contaminated hands before drinking water or food may be considered factors in the development of toxoplasmosis infection. According to the study performed by Assmar et al., the main route of toxoplasmosis infection in Iran is through soil and water (24). The highest relative frequency of Toxoplasma antibody titer was observed in Mazandaran province (20.5%) and the lowest frequency in Hormozgan province (2.9%). People aged 10 to 19 years showed a 50% increased risk of infection with a high antibody titer (24).
There was no significant relationship between the seroprevalence of toxoplasmosis and gender in working children and the control group. The seroprevalence of toxoplasmosis among the working children and control group in the age group of 11 - 14 years was higher compared to the age group of 7 - 10 years, and the difference was statistically significant among working children. Increasing the frequency of toxoplasmosis with age has been reported in previous studies (13, 25). In a study conducted by Sharif et al. in northern Iran in Sari, there was no significant relationship between the seroprevalence of toxoplasmosis and gender and age in children (13). However, the frequency of toxoplasmosis-positive cases increased with age (13). An increase in the frequency of toxoplasmosis-positive cases with increasing age has been previously reported by Dubey, which is due to exposure to toxoplasmosis due to increased life expectancy (25).
In the present study, IgM remained positive with lower titers in two children of the control group after three months; However, PCR results were negative. Although a positive IgM T. gondii antibody result indicates an acute infection, IgM antibody can remain for several months. Also, Toxoplasma false-positive results can be reported in IgM diagnostic test kits (26). A positive Toxoplasma IgM and IgG antibody result is not actually interpreted as an infection that recently occurred. The titers can be positive for months after an acute infection, so a complementary molecular assay is recommended for recent infections.
5.1. Conclusions
The seroprevalence of anti-T. gondii IgG antibody was higher in working children compared to the control group in Tehran. The present study showed a significant difference between working children and the control group regarding the frequency and titer of IgG antibodies. More exposure to the soil and contaminated hands before drinking water or food may be considered factors in the development of toxoplasmosis infection in these children.
5.2. Limitations
The study was performed on the children affiliated with The Sobh-e Rooyesh School, and the other population of working children was unavailable, which is a limitation of the study.