1. Background
In late December 2019, China reported a severe respiratory disease with a rapid spread among individuals. The World Health Organization (WHO) named it coronavirus disease 2019 (COVID-19) on February 11, 2020, and then declared it a worldwide pandemic (1). The clinical manifestations of COVID-19 are associated with respiratory and sometimes gastrointestinal symptoms (2). However, COVID-19 can damage tissues in other organs, including the heart, kidneys, endothelium, and the central nervous system (2). In severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and other infections by viruses from the beta-coronaviruses family, including SARS-COV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), the virus can access the central nervous system via various pathways such as the olfactory nerve axons and cause neuropsychiatric complications (3).
A review of previous respiratory epidemics reveals the high prevalence of mental disorders during and after the epidemic (4, 5). A meta-analysis study on the neuropsychological manifestations of acute coronavirus infections indicated the prevalence of disorders such as depression, insomnia, anxiety, memory impairment, and fatigue among patients with SARS and MERS (4). Compared to SARS and MERS, central and peripheral nervous system involvement is believed to be more different in COVID-19 (6, 7).
Despite the wide prevalence of COVID-19, few studies have examined its neuropsychiatric manifestations (7, 8). Moreover, these studies have focused on adult populations because of the lower prevalence of serious diseases in children and adolescents. Consequently, pediatricians have no clear understanding of the neurologic and psychiatric complications of COVID-19 in children and adolescents.
2. Objectives
This observational study aimed to evaluate neuropsychiatric complications in hospitalized children admitted for COVID-19 infection in Iran.
3. Methods
This prospective multi-center cross-sectional study included children and adolescents aged 4 - 18 years who were diagnosed with COVID-19 and admitted to the main referral children’s hospitals in eleven provinces of Iran as the representatives of Iran’s different geographical regions. Coronavirus disease 2019 is diagnosed based on polymerase chain reaction (PCR) tests and/or laboratory data and radiological findings. According to the Centers for Disease Control and Prevention (CDC) guidelines (9), the probable and confirmed cases of COVID-19 were included in this study from August to December 2021. Differential diagnoses were considered for probable cases and ruled out by lab data and other examinations. In Iran, decisions to admit children with COVID-19 are based on “An algorithmic approach to diagnosis and treatment of coronavirus disease 2019 (COVID-19) in children: Iranian experts’ consensus statement” (10). Confirmed or probable cases having high C-reactive protein, lymphocytopenia or thrombocytopenia, and lung involvement by chest X-ray or computerized tomography (CT) scan consistent with COVID-19 must have one of the following criteria for admission: Toxic or ill appearance, hemodynamic instability, refractory hypoxemia (O2 saturation < 92% despite oxygen therapy), acute hypercapnia or respiratory fatigue, decreased level of consciousness, history and physical examination consistent with lower respiratory tract involvement, concomitant co-morbidities (chronic lung diseases, cancer, immune deficiency, hemoglobinopathy or chronic kidney diseases). In this regard, patients with a past psychiatric history were excluded from this study. Informed consent was obtained from all caregivers and adolescents who were old enough to provide such consent.
Neurologic examination was performed after admission for all patients by pediatric neurologists. However, the patients were evaluated for psychiatric symptoms at the end of the first month of their COVID-19 manifestations. Child Behavior Checklist (CBCL), parent report was used as a screening instrument, and psychiatric evaluations were conducted for patients with psychiatric problems according to CBCL (11) by the child and adolescent psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders, Revision 5 (DSM-5). For children, there were clinical interviews with their parents; however, for the adolescents (11 - 18 years old), interviews were conducted with their parents and the patients themselves. To collect the required data, the researchers used a demographic checklist (gender, age, past medical history, PCR test result, illness duration, ICU admission, a multisystem inflammatory syndrome in children (MIS-C) status, fever, laboratory evidence of inflammation, and evidence of clinically-severe illness requiring hospitalization, with multisystem (> 2) organ involvement (12) and COVID-19 treatment and neurologic findings [consciousness level, gait, cranial nerves exam, muscle tone and force, deep tendon reflex, Babinski sign, electrodiagnostic study’s findings, cerebrospinal fluid (CSF), brain and spinal, magnetic resonance imaging (MRI) and electroencephalograms (EEG)].
This study was approved by the Research Ethics Committee of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (code: IR.SBMU.RICH.REC.1399.058, link: ethics.research.ac.ir/EthicsProposalView.php?id=171762).
3.1. Statistical Analysis
Statistical analysis was performed with SPSS software version 17 for windows (IBM Inc, NY). Descriptive statistics were used to summarize the data. Univariate analyses (the Mann-Whitney U test, independent t-test, Fisher’s exact test, and χ2) were used to evaluate the relationship between independent variables and the frequency of neurologic and psychiatric disorders. In this study, P < 0.05 was set as the significance level.
4. Results
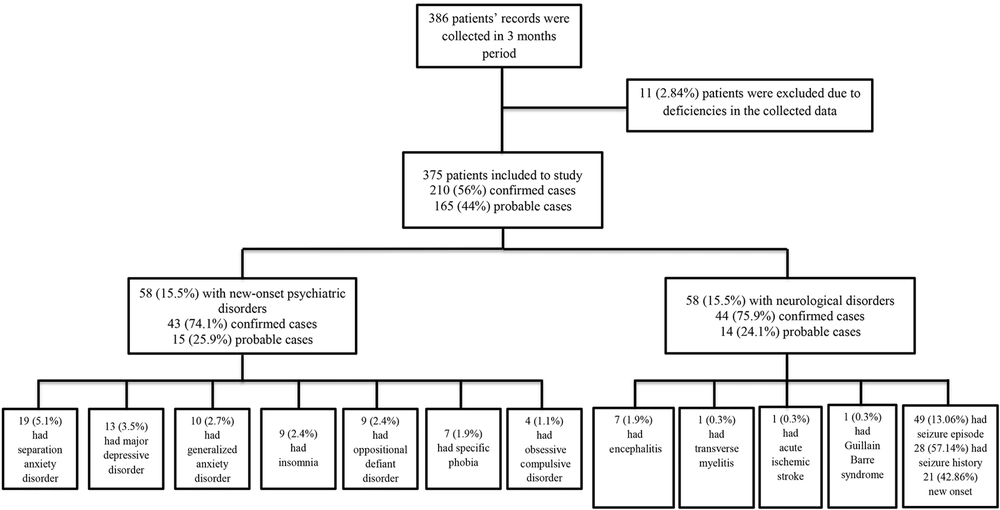
In this study, the participants encompassed 375 patients with a mean age of 9.00 ± 3.39 years, a majority (53.7%) of whom were in middle childhood (6 - 11 years). There were 20% of patients aged 4-5 years, and 26.3% aged 12 - 18 years, respectively. Of the 375 patients, 176 (47%) were female, and 199 (53%) were male. Forty-five percent (171/375) of the admitted patients had a medical history of neurological disorders (Appendix).
Of the 375 patients, 210 (56%) patients had the COVID-19 diagnosis based on reverse transcriptase-polymerase chain reaction (RT-PCR) results (confirmed cases), and 165 (44%) persons were diagnosed according to their clinical, laboratory, and radiologic manifestations compatible with COVID-19 (probable cases) because either they could not be tested (120, 32%) or their tests were negative (45, 12%).
Considering the duration of the disease (since symptoms onset), there were 128 patients (34.1%) with < 1 week, 68 patients (18.1%) with 1 - 2 weeks, 27 patients (7.2%) with 2 - 3 weeks, and 116 patients (30.93%) with > 3 weeks. There was no data available for 36 patients in this regard.
One hundred and three patients (27.5%) were admitted to ICU because of the severity of their symptoms. Seventy-two patients (19.2%) had MIS-C, and most of these patients (48/72, 66.7%) had positive PCR (P = 0.043). Regarding the COVID-19 treatment, corticosteroids (41.87%) was the most frequently received treatment, followed by antivirals (26.13%), intravenous immune globulin (IVIG) (24.8%), anti-malarial drugs (8%), antiepileptic (AEDs) drugs (6.4%), non-steroidal anti-inflammatory drugs (NSAIDS) (6.13%), Interferon (4.8%), sedatives (4.27%), and antitussives (0.53%).
The analysis revealed that 58 (15.5%) persons had psychiatric manifestations, and 58 (15.5%) subjects had neurologic complications (Figure 1). Moreover, three (0.8%) patients had both neurological and psychiatric symptoms.
4.1. Neurological Findings
Among the neurologic complications, the seizure was the most prevalent (49/375, 13.1%). In this study, 28 patients had a history of seizures, of whom 16 (57%) persons had a concurrent physical disorder. The other neurologic complications were encephalitis (1.9%), transverse myelitis (0.3%), acute ischemic stroke (0.3%), and Guillain-Barre syndrome (0.3%), respectively (Figure 1).
There were 21 patients with new-onset seizure attacks (with no positive history of seizures prior to COVID-19 infection). Almost all patients (n = 20) had seizures with fever (four patients with MIS-C, one patient with encephalitis/encephalopathy, and others with upper respiratory infection or gastroenteritis). Seizures in 11 patients (two with status epilepticus and nine with generalized tonic-clonic seizures) were controlled with anti-seizure medications; however, 10 patients had uncontrolled seizures following medications during the first days of infection. Most of the patients with new-onset seizures (14/21, 70%) were in the age group 6 - 11 years, and 10% of patients were below six years of age. Of the 21 patients with new-onset seizures, only two persons had abnormal neurologic history, and others had no neurological history or family history of seizures.
Most of the patients with seizures (35/49, 71.4%) had positive PCR for COVID-19 (P = 0.02), and there was no significant difference between the new-onset and history of the seizure groups (P = 0.52). There was no significant difference between patients with a history of seizure and new-onset seizures in terms of age, gender, prematurity, ICU admission, and ventilator use (Table 1).
| Factors | Patients with New-onset Seizure (n = 21) | Patients with a History of Seizure (n = 28) | P-Value |
|---|---|---|---|
| Age (y) | 9.25 ± 2.84 | 8.42 ± 3.46 | 0.38 |
| Gender (male) | 14 (66.7) | 16 (57.1) | 0.56 |
| Ventilator using at birth | 1 (4.8) | 0 | 0.42 |
| Prematurity | 3 (14.3) | 7 (25) | 0.48 |
| ICU admission | 6 (28.6) | 11 (39.3) | 0.54 |
a Values are expressed as mean ± SD or No. (%).
Forty-nine patients with seizures were evaluated by EEG, of whom ten patients had abnormal EEG, and all had a history of seizures (four patients with uncontrolled and six patients with controlled seizure history). The most prevalent findings in the EEG of patient with uncontrolled seizures were bursts of poly-spike discharges, multifocal spike-wave discharges, and definitely abnormalities caused by spike-wave discharges. However, in the controlled group, these abnormalities were not detected in the entire EEG tracing. The EEG results were normal in all patients with new-onset seizures.
Fourteen patients had abnormal brain MRI results, of whom eight patients had a history of epilepsy. In patients with a history of epilepsy, MRI abnormalities were as follows: Myelomeningocele and hydrocephalus (n = 1), leptomeningeal carcinomatous (n = 1), stroke (n = 1), cerebral edema due to diabetic ketoacidosis (n = 1), neurodevelopmental delay (n = 1), acute lymphocytic leukemia (n = 1), adrenoleukodystrophy (n = 1), and metachromatic leukodystrophy (n = 1). In patients with no history of epilepsy, MRI abnormalities were abnormal white matter hyperintensity (n = 3), ischemic stroke (n = 1), cerebral edema (n = 1), and abnormal hyperintensity in bilateral basal ganglia and cerebellar dentate (n = 1). Two patients had abnormal spinal MRIs due to cervical disc herniation (with cerebral edema) and transverse myelitis. In three cases, the abnormal spinal MRI was due to other neurological problems (two patients after tumor surgery in foramen magnum and one with acute ischemic total spinal cord caused by arteriovenous fistula thrombosis).
Among the five patients with loss of consciousness, there were two patients with, one patient with cerebral palsy and respiratory distress, one patient with a history of seizure and neurodevelopmental delay, and one patient with tramadol toxicity.
Abnormal neurology milestones were reported in 19 patients (19/375, 5%), of whom 10 (10/375, 2%) patients had gait abnormalities, and nine patients had a normal gait. Among the 356 normally-developed children, 27 persons had difficulty in walking, of whom seven patients could not walk due to failures in the central nervous system (two spinal tumors, one case of arteriovenous fistula in the spinal cord, one acute ischemic stroke, one cerebral edema, one encephalopathy, and one transverse myelitis). Among the 27 patients, MISC, seizure disorders, neurometabolic diseases (mitochondrial diseases), and diabetes mellitus (axonal peripheral polyneuropathy) were found as the possible causes of abnormal gait in six, four, one, and one case, respectively. The other patients could not walk with non-specific symptoms of weakness, lethargy, fever, nausea, vomiting, or back/limb pains. Walking problems in patients with normal developmental history have not been common in etiology, and further analysis of this group was not possible.
Abnormal nerve conduction study (NCS) and electromyography (EMG) address underlying diseases. One patient with axonal peripheral polyneuropathy had diabetes mellitus. Myopathic changes in EMG were detected in a patient with rheumatoid arthritis and one patient with renal failure (Table 2). Table 2 presents further details of neurologic findings.
| Neurologic Paraclinical Findings | New Signs/Symptoms or Paraclinical Findings After COVID-19 Infection | Signs/Symptoms or Paraclinical Findings Before COVID-19 Infection |
|---|---|---|
| Abnormal EEG | 0 | 10/49 (20.4) |
| Abnormal brain MRI | 6/24 (25) | 8/24 (33.3) |
| Abnormal spinal MRI | 2/17 (11.7) | 3/17 (17.6) |
| Abnormal EMG | 0 | 3/6 (0.8) |
| Abnormal NCS | 0 | 1/5 (0.27) |
| Abnormal CSF findings a | 8/42 (19) | 0 |
| Abnormal ABR | 0 | 3/43 (7) |
| Abnormal VEP | 0 | 1/38 (2.6) |
| Neurological exam findings | ||
| DTR a | ||
| Decreased | 17/375 (4.5) | - |
| Increased | 51/375 (13.6) | - |
| Positive Babinski sign a | 27/375 (7.2) | - |
| Abnormal sensory exam a | 3/375 (0.8) | - |
| Abnormal muscle tone a | 29/375 (7.7) | - |
| Hypotonic | 25/375 (6.6) | - |
| Hypertonic | 4/375 (1) | - |
| Decreased muscle force a | 32/375 (8.5) | - |
| Abnormal gait | 20/338 (5.9) | 17/338 (5) |
| Abnormal cranial nerves | 1/375 (0.2) | 12/375 (3.2) |
| Abnormal consciousness level | 5/375 (1.3) | 0 |
Abbreviations: COVID-19, Coronavirus disease 2019; VEP, visual evoked potential; DTR, deep tendon reflex; MRI, magnetic resonance imaging; EEG, electroencephalography; EMG, electromyography; NCS, nerve conduction study; CSF, cerebrospinal fluid; ABR, auditory brainstem response
a Variables in the physical exam probably existed prior to admission.
4.2. Psychiatric Findings
Fifty-eight (15.5%) patients had psychiatric disorders (Figure 1), including SAD (19, 5.1%), MDD (13, 3.5%), GAD (10, 2.7%), insomnia (9, 2.4%), ODD (9, 2.4%), specific phobia (7, 1.9%), and obsessive-compulsive disorder (OCD) (4, 1.1%). Due to the concurrence of psychiatric disorders in some patients, the frequency of the reported disorders exceeds the number of patients.
The mean age was 8.83 ± 2.52 for the patients with SAD, 10.78 ± 3.57 years for those with MDD, 11.44 ± 2.40 years for those with GAD, 10.12 ± 2.74 years for the ones with insomnia, 8.53 ± 3.62 years for the patients with ODD, 9.14 ± 3.05 years for those with a specific phobia, and 11.09 ± 2.15 years for those with OCD. There was no significant relationship between age and the incidence of psychiatric symptoms (P = 0.07).
There was no significant relationship between some other factors, including age, gender, duration of COVID-19 infection, ICU admission, history of COVID-19, and death from COVID-19 in the family, the history of neurologic problems, and single-parent family with the incidence of psychiatric symptoms (Table 3).
| Variables | Patients Without Psychiatric History (n = 213) | P-Value | |
|---|---|---|---|
| Patients with New Onset Psychiatric Disorders (n = 58) | Patients with Normal Psychology (n = 155) | ||
| Age (y) | 9.64 ± 3.39 | 8.67 ± 3.44 | 0.07 |
| Gender (male) | 32 (55.2) | 86 (55.5) | 0.96 |
| Prolonged duration of coronavirus disease 2019 (above 3 weeks) | 22 (38) | 69 (44.5) | 0.54 |
| History of coronavirus disease 2019 in family | 21 (36.2) | 55 (35.5) | 0.92 |
| Death from coronavirus disease 2019 in family | 4 (6.9) | 17 (11.0) | 0.37 |
| ICU admission | 20 (34.5) | 45 (29) | 0.44 |
| History of neurologic problems in patients | 2 (3.4) | 6 (3.9) | 0.88 |
| Patient’s caregiver | 0.24 | ||
| Parents | 54 (93.1) | 149 (96.1) | |
| Single parents or other guardians | 4 (6.9) | 6 (3.9) | |
The comparison of definite and probable cases with psychiatric manifestations revealed no significant difference in these groups.
5. Discussion
This study was the first comprehensive research on the neuropsychiatric complications of COVID-19 infection in hospitalized children and adolescents in the acute phase of this disease, conducted nationally in Iran. To the best of our knowledge, no other study has assessed the neuropsychiatric manifestations of COVID-19 in children referred for inpatient treatment nationally. Although there are several studies on the neuropsychiatric complication of COVID-19, they are review studies (4, 6, 13-17), case reports (1, 18, 19), and adult population studies (4) addressing neurological complications (17, 20, 21) or outpatient children (22). Accordingly, this study, for the first time, investigated neuropsychiatric manifestations in children and adolescents admitted for COVID-19 infection.
Similar to the previous studies on psychiatric and neurologic manifestations in adults (4, 8), the most prevalent neurologic and psychiatric complications among children and adolescents with COVID-19 infection in this study were seizures and anxiety/mood disorders. To date, there has been no report on seizure prevalence in pediatric patients with COVID-19; however, seizures were more common in this study in comparison to the normative rate of the general population (13% in our study vs. 3% - 5.5% in the Iranian population) (23). Anxiety/mood manifestations were more common in the present study compared to Mohammadi et al.’s study, and the prevalence of depressive disorders and SAD in Iranian children and adolescents were 1.4% and 1.53%, respectively (24).
Among the participants, 15.5% had psychiatric manifestations, and 15.5% had neurologic complications after COVID-19 infection. In adult studies, central nervous system involvement was more prevalent in severe cases than in non-severe ones (16). In the present study, the history of hematology and oncology disorders (n = 46) and the history of neurological problems (n = 46) were the most prevalent medical records (Appendix).
There was no abnormal EEG in the patients with new-onset seizures, and they revealed non-significant findings in EEG. Seizures in these patients seem to be caused by the fever and/or the viral infection in their central nervous systems; however, their EEGs were reported normal (25, 26). In our study, new-onset seizures were more common in the 6 - 11-year-old group, and COVID-19 infection played a significant role in the new-onset seizures in this group. According to Kurd et al., seizures may occur after COVID-19 infection without a previous history of seizure and are not associated with severe COVID-19-related illness (27). The same findings were observed in the present study.
In this study, most patients with seizures were confirmed COVID-19 cases with positive PCR tests; hence, COVID-19 was considered as another risk factor for febrile and afebrile seizures in the youth (27). Some studies have discussed the potential neurotropism of COVID-19 and suggested that the neuropsychiatric manifestations in the pre-existing patient were more prevalent and more severe (15, 16). The prevalence of other neurologic complications (i.e., encephalitis, transverse myelitis, acute ischemic stroke, and Guillain-Barre syndrome) was similar to recent studies on adult patients; however, less severe neurological complications were observed in children and adolescents (1, 7, 8, 17).
Previous studies have indicated that COVID-19 infection may increase psychiatric disorders such as depression and anxiety (4). However, to the best of our knowledge, no similar study has addressed psychiatric manifestations in admitted COVID-19 children and adolescents. In the present study, psychiatric manifestations were reported in 58 patients (15.5%), and the most common psychiatric disorders were SAD, MDD, GAD, insomnia, and ODD. In a cross-sectional study on two institutional quarantine centers in Qatar, Khan et al. reported anxiety symptoms in 36.03% of children with COVID-19 infection, of whom about 70% and 58% had physical injury fears and SAD, respectively (28). Although our findings are related to admitted COVID-19 patients, SAD was the most common in both studies. These findings are consistent with those reported by Mazza et al., as they reported high levels of anxiety and depression in individuals recovering from hospitalization for COVID-19 (29). In line with the present study, in Cai et al.’s study, 28 (22.2%) and 48 (38.1%) patients received cut-off points for the clinically significant symptoms of anxiety and depression after discharge, respectively (30). Moreover, Taquet et al. reached similar findings (31).
In a study on adult cases with COVID-19, the prevalence of neuropsychiatric complications was 22.5%. The most common psychiatric manifestations were anxiety (4.6%) and mood disorders (3.8%), respectively (8). In line with the present study, Rogers et al. conducted a systematic review and meta-analysis on psychiatric complications in patients with COVID-19 (4). The study demonstrated that anxiety and depression were the most common psychiatric complications caused by severe coronavirus infections in all age groups (4).
The mean age of the patients in our study was nine years, and there was no significant relationship between the age (P = 0.07), with the incidence of psychiatric symptoms. While in the UK study, more than half of the patients were 71 years old. Also, in the study of Nalleballe et al., most of the patients were between 18 and 50 years old (8). More studies are needed to evaluate the aging effect in the children group.
The frequency of psychiatric symptoms was not high in patients with a prolonged duration (above three weeks) of COVID-19 (P = 0.54). According to previous studies, some neuropsychiatric symptoms may occur in critically-ill survivors after ICU admission (14, 32). The present findings revealed no significant relationship between ICU admission and the incidence of psychiatric symptoms in children with COVID-19 (P = 0.44). In line with the present findings (P = 0.37) (Table 3), Slomski reported that most children and caregivers who experienced their loved one’s death from COVID-19 had resilience in the face of grief (33).
In general, several mechanisms can be proposed to explain the observed neuropsychiatric symptoms, such as systemic inflammation and released inflammatory cytokines, treatment with high-dose corticosteroids, pandemic-related family burden, reaction to illness and hospitalization, and poor coping styles in patients (6, 34-36).
According to some studies, early intervention in psychiatric disorders is associated with better outcomes; hence, detecting psychiatric symptoms in the early stages for high-risk patients could provide appropriate early interventions. Psychiatric disorders are both risk factors and the consequences of infection with COVID-19 (37, 38); hence, the patients suffering from such disorders should be considered, with other patients prioritized for vaccination (39).
This research probably is the first study focusing on the neuropsychiatric manifestations of children and adolescents admitted for COVID-19. Regarding the research limitations, since there was no comparison group, it was difficult to determine whether the infection caused these psychiatric symptoms or whether other causes were involved. Moreover, psychiatric disorders in parents were not assessed in this study. However, parents are an essential risk factor for psychiatric problems in patients. The short research period was another limitation. Furthermore, the neurological examination was only considered on admission for the studied cases, and the CNS complications, which possibly developed later in the course of hospitalization, were not addressed. Additional studies with the control group and prolonged follow-up would provide more reliable information. Moreover, future researchers are recommended to conduct follow-up studies and compare findings for patients in other countries.
5.1. Conclusions
The present study indicates that the neuropsychiatric manifestations in children and adolescents admitted for COVID-19 are not as common as expected, among which seizure and anxiety disorders are the most prevalent.