1. Background
Urinary tract infections (UTIs) are among the common childhood infections. Approximately 8% of girls and 2% of boys develop UTIs by the age of 11 years. Generally, UTIs are more common in boys under the age of one year but more frequent in girls afterward (1). About 90% of newly-diagnosed UTIs and 75% of recurrent UTIs are caused by Escherichia coli, which is a bacterium in the normal gut flora, ascending from the intestinal tract to colonize in the urinary tract. Other bacteria causing UTIs include Klebsiella spp., Proteus spp., Enterococcus spp., Pseudomonas spp., and Staphylococcus saprophyticus (2, 3). The signs and symptoms of UTIs vary greatly depending on the age of the child. In infants, the most common symptoms are low birth weight, breastfeeding difficulties, indirect hyperbilirubinemia, and fever. Furthermore, from the age of one month to two years, breastfeeding difficulties, infantile colic, poor weight gain, diarrhea, vomiting, and unexplained fever comprise the most common causes of referral to the hospital. Regardless of the initial complaints and age, UTIs should be suspected in all feverish patients presenting with urinary tract abnormalities (4).
The diagnosis of UTIs in infants and young children requires both pyuria and identifying at least 50,000 CFU/mL of at least one uropathogenic bacterial strain in urine culture. A leukocyte count greater than 10/µL of urine can be a sign of an infection; however, pyuria can also be seen in urethritis, vaginitis, urinary stones, glomerulonephritis, and interstitial nephritis. A strip test containing leukocyte esterase and nitrite (i.e., positive leukocyte esterase and microscopically identified bacteria) provides 70% sensitivity and 99% specificity for the diagnosis of UTIs. The use of either of these two diagnostic parameters alone offers a very low sensitivity (5, 6). Before starting antibiotic therapy, it is necessary to obtain an impeccable urine sample from the patient (i.e., a midstream sample via either urinary catheter or bladder aspiration) and send it for analysis and culture within a maximum of one hour. The course of antibiotic therapy for cystitis is 5 days, and for pyelonephritis is 10 to 14 days (2, 7). Empirical treatment with broad-spectrum antibiotics can be started to target main UTI-causing bacteria such as E. coli, Proteus spp., and Klebsiella spp. until the culture result is ready. The possibility of antibiotic resistance should always be considered, especially when administering ampicillin or other antibiotics to which resistance has been reported in recent years (7, 8).
Pediatric UTIs are important because of their potential risks, complications of inappropriate antibiotic therapy, and frequent changes in antibiotic susceptibility and resistance patterns, which may have irreversible implications for both the patient and the healthcare provider. Physicians should monitor the common bacteria that cause UTIs and periodically evaluate their antibacterial susceptibility and resistance patterns (usually every 5 years) in any geographic area or even any medical center. Identifying the most common bacteria that cause UTIs, as well as the prevalence of antibacterial susceptibility and resistance, will allow patients to be treated with the most effective antibiotics with the fewest side effects.
2. Objectives
The aim of this study was to evaluate the prevalence and antibiotic resistance patterns of uropathogenic bacteria causing UTIs in children under 3 years of age admitted to the 17th Shahrivar Hospital of Rasht City, north of Iran.
3. Methods
This cross-sectional descriptive study was conducted on children younger than 3 years old presenting with lower or upper UTIs hospitalized in our hospital from 2014 to 2020. The ethical approval code was received from the ethics committee of Guilan University of Medical Sciences (IR.GUMS.REC.1398.420). In this study, the children were primarily controlled for eligibility criteria, underwent a complete physical examination, including axillary temperature measurement at admission, administered with appropriate treatments, and finally, the study questionnaire was completed for them.
Then a mid-stream urine sample (after washing the perineal area) was obtained from children who had urinary control; in children who did not have urinary control, either the suprapubic or catheter method was preferably used, and in the case of failure, urine samples were collected from a urine bag. The samples were immediately sent to the medical laboratory located on the 17th Shahrivar Children's Hospital for urine analysis and culture. In this study, the children were divided into two groups based on two variables: Age and hospitalization period. For the variable of age, the children were divided into two groups: Those younger than 60 days and those older than 60 days. For the variable of hospitalization period, the children were divided into two groups: Those hospitalized for less than 7 days and those hospitalized for more than 7 days. This division was done to determine the prevalence of uropathogenic bacteria in each group and to analyze any potential differences based on age and hospitalization duration. In this study, either pyuria or a positive nitrite test was considered the primary evidence of an active infection. The growth of more than 104 or 103 bacteria on Muller Hinton Agar in urine samples collected from urine bags or through a urinary catheter, respectively, was considered a positive culture. Any bacterial growth in suprapubic samples was considered a positive culture.
Children with chronic UTIs or anatomical disorders of the urinary system were not included in this study. Also, children with a history of previous hospitalizations or taking antibiotics for any reason were excluded from the study.
The variables gathered in this study were age, gender, paraclinical results (such as leukocyte count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and nitrate), and prevalence of isolated uropathogenic bacteria, as well as their antibiotic resistance patterns. For urinalysis, 1 mL of samples was centrifuged for 15 minutes (1,000 - 2,000 rpm) at room temperature. Then the clear supernatant was removed, and the sediment was evaluated for identifying and enumerating white blood cells, red blood cells, epithelial cells, bacteria, hyaline casts, and abnormal crystals.
An increase in the number of white blood cells (WBCs) in urine, known as pyuria, is commonly observed in urinary tract infections (UTIs). In cases of acute pyelonephritis, an elevated number of WBCs is accompanied by the presence of protein and bacteria in the urine. Lower UTIs also result in an increased number of WBCs in urine but with only slight levels of protein. Leukocyturia (LU), which refers to the presence of leukocytes in urine, can be caused by urinary infections or non-infectious factors. For urine culture, 0.1 mL of loop-impregnated urine samples was cultured on blood agar and EMB (Merck-Germany) medium and incubated at 37°C for 24 - 48 hours. In our study, positive urine culture was defined as the growth of more than 100,000 or 50,000 colonies from a uropathogenic bacterium in urine samples collected through a catheter or via suprapubic sampling, respectively (9).
Shedding of greater than two million leukocytes in urine per day is called leukocyturia (10, 11). All bacterial species were identified by direct smearing and biochemical tests (12).
Antibiogram was determined using a phenotypic-based technique in which microbial growth was examined in the presence of a variety of antibiotics. These techniques include agar dilution (gold standard), broth microdilution, microdilution, and antibiotic-gradient strips. In fact, antibiotic susceptibility testing is an in vitro test to ascertain the sensitivity of a bacterium to one or more antibiotics by the diffusion technique on agar media. Its purpose is to guide the clinician when choosing an antibiotic to treat a bacterial infection and to monitor bacterial resistance to antibiotics. Mueller-Hinton agar (Merck-Germany) was used to determine antibiogram based on CLSI guidelines. Antibiotic discs used in this study included ciprofloxacin, nalidixic acid, amikacin, gentamicin, imipenem, cephalothin, cotrimoxazole, cefotaxime, nitrofurantoin, norfloxacin, cephalexin, ampicillin, ceftriaxone, amoxicillin, tetracycline, carbenicillin, ofloxacin, and cefixime (PadtanTeb Co., Iran) (13).
In order to measure ESR, standard tubes were filled with blood samples and positioned on a rack in a completely vertical position for 1 hour at room temperature. Then the distance from the lowest point of the surface meniscus to the upper limit of the red cell sediment was measured and expressed as millimeters in 1 hour.
A test based on latex agglutination was used to measure CRP. When latex particles form complexes, human anti-CRP antibodies are mixed with the patient’s serum containing CRP. A visible agglutination reaction will take place within 2 minutes. Before performing the test, all reagents and serum samples were brought to room temperature and gently mixed with the latex reagent prior to use. One drop of each of the sera samples, positive control sample, and negative control sample was placed on a separate reaction circle on a glass slide. Then1 drop of the CRP latex reagent was added to each of the circles. Next, separate sticks were used to mix the samples, and the spreader fluid was poured all over the circle. The slide’s back was shaken slowly for 2 minutes and visualized under artificial light.
Further testing was performed using a dipstick containing a reagent reacting with nitrites, generating pink color, thus indirectly suggesting the presence of bacteria. A positive test result indicates the need for a urine culture. Nitrite screening enhances the sensitivity of the leukocyte esterase test in detecting UTIs.
In order to count the number of bacterial colonies on the Muller Hinton agar culture medium, urine samples were initially cultured using the spread plate method. Then plates carrying over 200 colonies were counted by dividing the plate’s area into equal sectors (from 1/2 up to 1/8). After counting one sector, the count was multiplied by the total number of sectors to estimate the number of colonies on the whole plate.
3.1. Statistical Tests
To determine the prevalence of the bacteria causing UTIs, as well as their microbial resistance, frequency, frequency percentage, and 95% confidence intervals were used. Either the chi-square test or Fisher’s exact test was used to compare the frequency of UTIs and microbial resistance by sex and age groups. The significance level of the tests was considered p-value < 0.05.
4. Results
Two hundred and fifty-nine children admitted to the 17th Shahrivar Hospital in Rasht, Iran, were included in the study, 77.2% of whom were less than 60 days old. The mean age of the patients was 4.9 ± 2.7 months, with a minimum age of one day and a maximum age of 36 months. The number of boys (53.3%) was higher than girls, and 59.5% of the children studied spent more than seven days in the hospital. The mean duration of hospitalization days was 7.96 ± 2.93 days; the minimum length of hospital stay was two days, and the maximum length of hospital stay was 23 days.
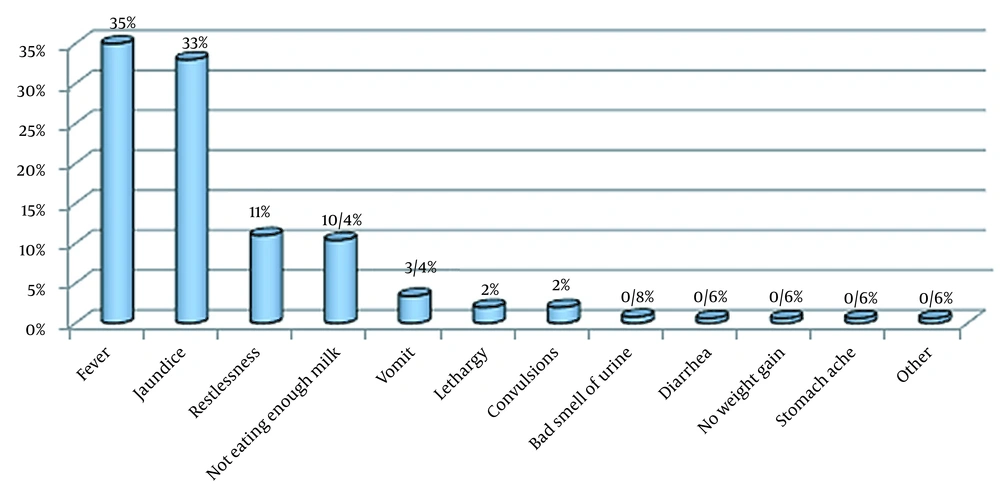
Among 259 children with UTIs, 346 clinical signs were found (some patients had two or more clinical signs). The most frequent symptom was fever (35%), followed by jaundice (33%), restlessness (11%), not drinking enough milk (10.4%), and vomiting (3.4%) (Figure 1).
Regarding clinical symptoms by age at the time of admission, jaundice, not drinking enough milk, and fever were the most common accompanying symptoms in children under 60 days. In children older than two months, fever and restlessness were more common than other symptoms. Leukocyturia was observed in 64.9% of the patients. The erythrocyte sedimentation rate was assessed in 105 patients, showing an increase in 98.1% of the cases. In addition, the mean ESR value in the studied samples was 38.61 ± 36.79 mm/h. C-reactive protein was tested in 211 children and was positive in 42.7% of them. Nitrate strip testing was negative in 75.4% of the children (Table 1).
| Laboratory Indices and Condition | Total | No. (%) |
|---|---|---|
| Leukocytes | ||
| Positive | 259 | 168 (64.9) |
| Negative | 259 | 91 (35.1) |
| ESR | ||
| Positive | 251 | 103 (98.1) |
| Negative | 251 | 2 (1.9) |
| ESR | ||
| Less than 20 | 251 | 47 (45.6) |
| 100 - 20 | 251 | 49 (47.6) |
| More than 100 | 251 | 7 (6.8) |
| CRP | ||
| Positive | 211 | 90 (42.7) |
| Negative | 211 | 121 (57.3) |
| Nitrate | ||
| Positive | 248 | 61 (24.6) |
| Negative | 248 | 187 (75.4) |
Abbreviations: ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
The most common isolate in patients’ urine cultures was E. coli, comprising 56.4% of all cases, followed by Klebsiella spp. (33.2%) (Table 2).
| Causes of UTIs | No. (%) | ||
|---|---|---|---|
| Age Less Than 60 Days | Age Above 60 Days | Total (N = 259) | |
| Escherichia coli | 102 (51) | 44 (74.6) | 146 (56.4) |
| Klebsiella spp. | 72 (36) | 14 (23.7) | 86 (33.2) |
| Enterobacter spp. | 18 (9) | 1 (1.7) | 19 (7.3) |
| Pseudomonas spp. | 4 (2) | 0 (0) | 4 (1.5) |
| Staph. aureus | 2 (1) | 0 (0) | 2 (0.8) |
| Citrobacter spp. | 2 (1) | 0 (0) | 2 (0.8) |
| Total | 200 (100) | 59 (100) | 259 (100) |
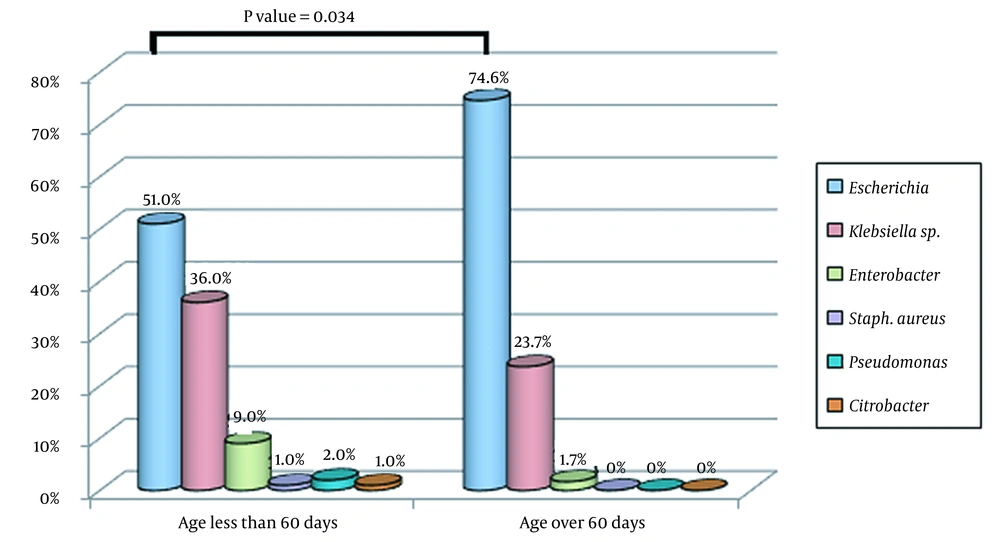
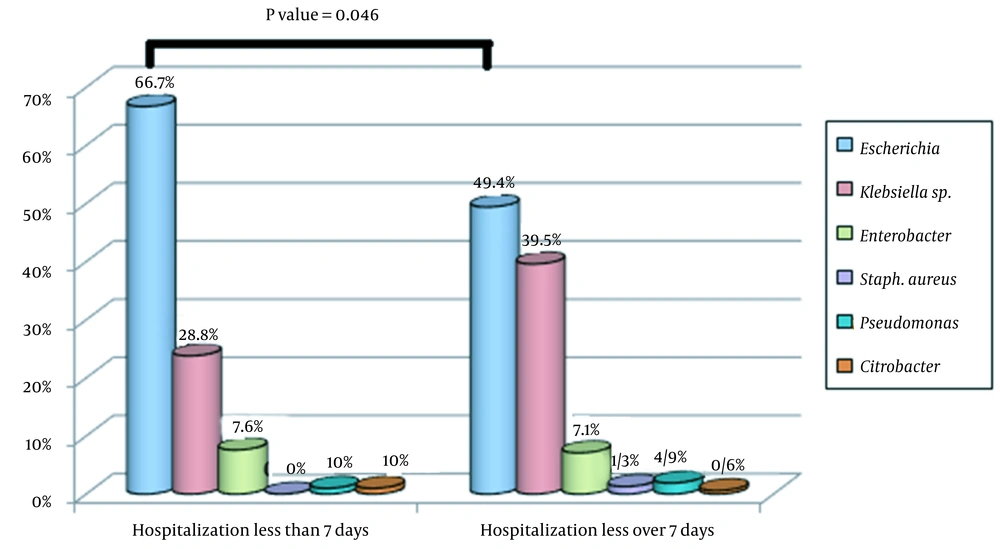
Figure 2 shows the frequency distribution of uropathogenic bacteria in children under three years of age diagnosed with UTIs based on age groups (less than 60 days and over 60 days). There was a statistically significant difference in the prevalence of uropathogenic bacteria causing UTIs between children younger or older than 60 days (P-value = 0.034, Figure 2). Also, Figure 3 displays the frequency distribution of uropathogenic bacteria causing UTIs based on the duration of hospitalization (less than or more than 7 days). There was a statistically significant relationship between the duration of hospitalization and the causative agent of UTIs (P-value = 0.046), and children with UTIs caused by Klebsiella spp. and Enterobacter spp. had longer hospitalization periods than children with infections caused by E. coli. (Figure 3).
Table 3 demonstrates the frequency distribution of antibiotic resistance patterns in children under 3 years of age presenting with UTIs according to the type of uropathogenic strains, showing that Klebsiella spp. and E. coli had the highest percentage of antibiotic resistance to nalidixic acid, gentamicin, cephalothin, cotrimoxazole, cefotaxime, and cephalexin. Table 4 shows the distribution of antibiotic susceptibility or antibiotic resistance in uropathogenic bacteria causing UTIs in children under 3 years of age. Ciprofloxacin, nitrofurantoin, amikacin, gentamicin, imipenem, and nalidixic acid showed the highest antibiotic susceptibility to uropathogens isolated from < 3-year-old children with UTIs. Also, cephalothin, cephalexin, ampicillin, and amoxicillin showed the highest antibiotic resistance to uropathogens isolated from these children.
| Antibiotics | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Antibiogram | Klebsiella spp. | Enterobacter spp. | Escherichia coli | Staphylococcus aureus spp. | Pseudomonas spp. | Citrobacter spp. | |
| Ciprofloxacin | Resistant | 6 (3.92) | 0 (0) | 7 (4.57) | 0 (0) | 0 (0) | 0 (0) |
| Nalidixic acid | Resistant | 22 (10.28) | 5 (2.33) | 51 (23.83) | 0 (0) | 0 (0) | 1 (0.46) |
| Amikacin | Resistant | 25 (14.79) | 2 (0.93) | 12 (7.1) | 0 (0) | 0 (0) | 0 (0) |
| Gentamicin | Resistant | 31 (14.97) | 4 (1.93) | 19 (9.17) | 0 (0) | 0 (0) | 0 (0) |
| Imipenem | Resistant | 1 (3.44) | 0 (0) | 8 (27.58) | 0 (0) | 0 (0) | 0 (0) |
| Cephalothin | Resistant | 48 (31.37) | 7 (4.57) | 61 (39.86) | 0 (0) | 1 (0.65) | 1 (0.65) |
| Cotrimoxazole | Resistant | 36 (19.67) | 4 (2.18) | 54 (29.5) | 0 (0) | 4 (2.18) | 0 (0) |
| Cefotaxime | Resistant | 32 (20.12) | 4 (2.51) | 50 (31.44) | 0 (0) | 1 (0.62) | 1 (0.62) |
| Nitrofurantoin | Resistant | 15 (6.55) | 5 (2.18) | 12 (5.24) | 0 (0) | 0 (0) | 0 (0) |
| Norfloxacin | Resistant | 1 (0.9) | 1 (0.9) | 10 (9.09) | 0 (0) | 0 (0) | 0 (0) |
| Cephalexin | Resistant | 18 (25.71) | 4 (5.71) | 29 (41.42) | 0 (0) | 3 (4.28) | 0 (0) |
| Ampicillin | Resistant | 18 (27.27) | 0 (0) | 31 (46.96) | 2 (3.03) | 0 (0) | 1 (1.51) |
| Ceftriaxone | Resistant | 15 (17.24) | 5 (5.74) | 20 (22.98) | 0 (0) | 0 (0) | 0 (0) |
| Amoxicillin | Resistant | 0 (0) | 1 (16.66) | 6 (100) | 0 (0) | 0 (0) | 0 (0) |
| Tetracycline | Resistant | 3 (20) | 1 (6.66) | 2 (13.33) | 0 (0) | 0 (0) | 1 (6.66) |
| Carbenicillin | Resistant | 0 (0) | 2 (18.18) | 5 (45.45) | 0 (0) | 0 (0) | 0 (0) |
| Oflaxin | Resistant | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) |
| Cefixime a | Resistant | 0 (0) | 2 (50) | 2 (50) | 0 (0) | 0 (0) | 0 (0) |
a Due to the hospitalization of patients and the need for injectable antibiotics, the number of antibiograms containing cefixime was limited.
| Antibiotics | Antimicrobial Resistance (AMR) | |
|---|---|---|
| Total (N) | No. (%) | |
| Ciprofloxacin | 153 | 13 (8.5) |
| Norfloxacin | 110 | 12 (10.9) |
| Ofloxacin | 8 | 1 (12.5) |
| Nitrofurantoin | 229 | 33 (14.4) |
| Amikacin | 169 | 39 (23.1) |
| Gentamicin | 207 | 54 (26.1) |
| Imipenem | 29 | 9 (31) |
| Nalidixic acid | 214 | 79 (37) |
| Ceftriaxone | 87 | 40 (46) |
| Tetracycline | 15 | 7 (47) |
| Cotrimoxazole | 183 | 98 (53.6) |
| Cefotaxime | 159 | 88 (55.3) |
| Carbenicillin | 11 | 7 (63.6) |
| Cephalothin | 153 | 118 (77.1) |
| Cephalexin | 70 | 54 (77.1) |
| Ampicillin | 66 | 52 (79) |
| Amoxicillin | 6 | 6 (100) |
| Cefixime a | 4 | 4 (100) |
a Due to the hospitalization of patients and the need for injectable antibiotics, the number of antibiograms containing cefixime was limited.
5. Discussion
In this study, we investigated children with positive urine cultures admitted to the 17th Shahrivar Hospital of Rasht City, Iran. Among 259 children under 3 years of age hospitalized with a diagnosis of UTIs, 200 children were less than 60 days old. About 59% of the children were hospitalized for more than 7 days, and the average duration of hospitalization was 7.96 days.
The most common clinical symptoms in our children were fever and restlessness. Fever has been reported in several studies as a common symptom in children hospitalized due to UTIs (14-17). It should be noted that most of these studies have been performed on hospitalized patients, who generally show a higher prevalence of upper UTIs and fever. Neonatal jaundice can also be the first sign of UTIs in infants and neonates before the appearance of other symptoms, so urine analysis is recommended to check for jaundice in asymptomatic icterus infants (14). According to our study, among 102 infants with jaundice in the first two weeks of life, UTIs were diagnosed in about 8%, highlighting the importance of urine analysis in all infants with unknown causes of jaundice for more than 3 days (9). In other studies involving infants who experienced prolonged jaundice lasting more than two weeks, the reported prevalence of urinary tract infections (UTIs) ranged from 5.8% to 6%. (9, 10, 14).
In the current study, ESR was assessed in 105 patients, and it was found to be elevated in 98.1% of the cases. This suggested that most of these patients had elevated ESR levels, indicating the presence of inflammation or an underlying condition. The mean ESR value in the studied population was 38.61 ± 36.79 mm/h, indicating elevated ESR levels. The wide standard deviation suggested that there was considerable variability in ESR values among these patients. C-reactive protein was tested in 211 of the patients and returned positive in 42.7% of the cases. A positive CRP result indicates the presence of acute inflammation or infections. The lower percentage of positive CRP compared to elevated ESR reflects the lower sensitivity of CRP in detecting inflammation compared to ESR in this study. Evidence indicates that elevated CRP and ESR can be associated with multidrug-resistant UTIs. As an acute-phase protein, CRP is produced by the liver in response to inflammation and is commonly used as a marker of systemic inflammation. Studies have shown that CRP levels are often elevated in patients with UTIs, and this elevation can be even more pronounced in patients with multidrug-resistant UTIs. Elevated CRP levels also predict a more severe and prolonged inflammatory response. Similarly, ESR is a non-specific marker of inflammation that measures the rate at which red blood cells settle in a tube containing anticoagulated blood. Similar to CRP, ESR can be elevated in patients with UTIs, particularly in those with multidrug-resistant infections. However, it is important to note that ESR can be influenced by various factors other than inflammation. Although simultaneously elevated CRP and ESR levels can be indicative of a more severe and resistant UTI, they are not definitive diagnostic markers for multidrug resistance. The gold standard for detecting multidrug-resistant UTIs is antimicrobial susceptibility testing. It is important to consult with a healthcare professional for proper evaluation and interpretation of CRP and ESR levels in the context of a suspected multidrug-resistant UTI.
In our study, 53.3% of the patients were boys. The incidence of UTIs is higher in male infants, especially uncircumcised boys (11). In some studies, no significant difference was reported in the incidence of UTIs between the sexes, and other studies have reported a higher prevalence of UTIs in girls than in boys, especially at the age of one year and older (12, 13, 18). Various factors, such as the structure and anatomy of the urinary tract (short urethra), can explain the higher prevalence of UTIs in girls. In addition, the close relationship between girls’ urinary systems and fecal microorganisms can be another reason for this observation (15). Given that UTIs among infants are more common in males than in females, and considering that many older children are treated on an outpatient basis, the overall higher number of boys in this study can be justifiable because 200 out of 259 children studied here were less than 60 days old.
Regarding the frequency distribution of uropathogenic bacteria in children under three years of age presenting with UTIs in different age groups (i.e., < 60 days and > 60 days) or hospitalization periods (< 7 days and > 7 days), uropathogenic bacteria causing UTIs were significantly associated with age (P-value = 0.034) and duration of hospitalization (P-value = 0.046). We observed that the average hospitalization period was longer in children with UTIs caused by Klebsiella spp. and Enterobacter spp. than children who suffered from E. coli infections. This can be due to the antibiotic resistance of Klebsiella spp. and Enterobacter spp., leading to unresponsiveness to standard antibiotic treatments.
In addition, the predominant pathogen causing UTIs in children less than or beyond 60 days old was E. coli, which was also the predominant pathogen in both girls and boys. In the study of Aghamahdi et al., E. coli (59.7%) and then Klebsiella spp. (10%) and Enterobacter spp. (14.3%) were the most common organisms isolated (16). In another study by Karimpour and Mohamadi E. coli was also identified as the cause of 71.1% of UTIs, and similar to our study, this bacterium was the most common causative organism in both sexes. In a recent study, the highest frequency after E. coli was related to Enterobacter spp. (17). According to Bay and Anacleto, E. coli had the highest frequency among other microorganisms isolated from urine cultures of UTI patients (11). In the study of Yousefimashouf and Molazadeh, E. coli was the cause of 64.3% of UTIs, followed by Klebsiella spp. as the second most common microorganism. As can be seen, in almost all studies, E. coli was the most common pathogen causing UTIs in children. However, there is slight variability in the frequency of pathogens besides E. coli, which may be related to differences in the distribution of pathogens in different geographical areas, even within a single country (19).
A large number of isolates causing UTIs in this study were resistant to cephalothin, cephalexin, ampicillin, and amoxicillin, but most of them, such as E. coli, Klebsiella spp., and Enterobacter spp., were sensitive to ciprofloxacin, nitrofurantoin, amikacin, gentamicin, imipenem, and nalidixic acid. Given that E. coli was the most common pathogen causing UTIs, the results of our study suggest that E. coli can be reasonably sensitive to ciprofloxacin, amikacin, gentamicin, nitrofurantoin, imipenem, ceftriaxone (59.2%), and nalidixic acid. Therefore, it seems that aminoglycosides and ciprofloxacin are the best agents to be used for the empirical treatment of children admitted to our hospital due to UTIs. In a study by Prakasam et al., E. coli was most sensitive to ciprofloxacin, followed by amikacin, but this bacterium was not desirably sensitive to cotrimoxazole and cefotaxime (20). Barzan et al. reported that E. coli was sensitive to amikacin, tazobactam, and nitrofurantoin and had the highest resistance to cephalothin and cotrimoxazole (12). Aghamahdi et al. showed that their isolated uropathogenic strains were resistant to ampicillin, amoxicillin, cephalexin, cotrimoxazole, and cefixime by the respective rates of 94.1%, 88.9%, 70%, 66.7%, and 75%; however, the rate of antibacterial resistance was lower against nalidixic acid, ceftriaxone, aminoglycosides, nitrofurantoin, and ciprofloxacin (16). In comparison, the rate of resistance to cotrimoxazole was lower (66.7% vs. 53.6%), while resistance to ceftriaxone was higher (20% vs. 46%) in our study. This discrepancy seems to be due to the administration of ceftriaxone during this period (16). In a study by Valavi et al. in Ahvaz, E. coli had the highest resistance to cotrimoxazole and the lowest resistance to nitrofurantoin (21).
The choice of antibiotics to eradicate multidrug-resistant uropathogens, such as Pseudomonas aeruginosa, Enterobacteriaceae spp., and Staphylococcispp., depends on several factors, including local resistance patterns, patient-specific factors, and severity of the infection. It is important to note that antibiotic recommendations can vary across different regions and healthcare facilities, and it is crucial to consult local guidelines and infectious disease specialists to choose appropriate antibiotics. In general, combination therapy may be considered for multidrug-resistant uropathogens to improve treatment efficacy and reduce the risk of further resistance. The specific combination of antibiotics will depend on the bacteria isolated and their antibiotic susceptibility patterns. For P. aeruginosa, which is known for its multidrug resistance, combination therapy with two or more antibiotics from different classes is often recommended. Commonly used antibiotics for P. aeruginosa include carbapenems (e.g., meropenem and imipenem), antipseudomonal penicillin (e.g., piperacillin-tazobactam), cephalosporins (e.g., ceftazidime and cefepime), and fluoroquinolones (e.g., ciprofloxacin and levofloxacin) (22). However, susceptibility testing guide better in the choice of antibiotics. For Enterobacteriaceae spp., including E. coli and K. pneumoniae, the choice of antibiotics may depend on the specific resistance mechanisms detected. Carbapenems (e.g., meropenem and imipenem) are often considered the drugs of choice for extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae spp. Other options may include combinations of newer beta-lactam/beta-lactamase inhibitors (e.g., ceftazidime-avibactam) or polymyxins (e.g., colistin) (23). Again, susceptibility testing is important for choosing appropriate antibiotics. For Staphylococci, including methicillin-resistant Staphylococcus aureus (MRSA), treatment options may encompass vancomycin, daptomycin, linezolid, or newer agents such as cefazoline or tedizolid (24). Susceptibility testing should be performed to determine the most effective antibiotic. It is crucial to note that the choice of antibiotics should be based on local resistance patterns, individualized patient-related factors, and guidance from infectious disease specialists. The regular surveillance of antimicrobial resistance patterns is important to be informed of empirical therapy choices and ensure optimal therapeutic outcomes (25).
Antibiotic resistance is a significant concern when treating pediatric UTIs. Empirical treatment should be based on the local prevalence and patterns of antibiotic resistance. Although E. coli is the most commonly isolated pathogen in UTIs, it is important to note that the susceptibility patterns of this bacterium can vary between regions and change over time. However, based on the susceptibility results observed in the present study, E. coli showed susceptibility to ciprofloxacin, nitrofurantoin, amikacin, gentamicin, and nalidixic acid. Considering the potential side effects of ciprofloxacin in children, it is reasonable to consider alternative antibiotics with suitable therapeutic effects and fewer side effects, such as aminoglycosides (e.g., amikacin and gentamicin). These antibiotics can be effective in controlling potentially drug-resistant pediatric UTIs. Nitrofurantoin and nalidixic acid have also been mentioned as appropriate choices for treating lower UTIs (e.g., cystitis) but not upper UTIs and pyelonephritis due to their limited tissue penetration. It is important to consider the age of the children being treated. As mentioned in the present study, different age groups may have different susceptibility patterns, requiring necessary adjustments in therapeutic approaches.
We should acknowledge some limitations of our study, including the limited age groups studied. There is a need for a larger multicenter study to obtain more valuable and generalizable results. Conducting multicenter studies can provide a broader perspective on regional variations in antibiotic resistance patterns and guide healthcare professionals in choosing appropriate empirical treatments. In summary, the choice of empirical treatment for pediatric UTIs should be based on the local prevalence and patterns of antibiotic resistance. Regular surveillance of resistance patterns is crucial to make informed treatment decisions and ensure optimal clinical outcomes in children with UTIs.
5.1. Conclusions
It is recommended to use aminoglycosides as the first-line drugs for the empirical treatment of pediatric UTIs. If aminoglycosides cannot be used due to contraindications, third-generation cephalosporins can be considered. If there is no improvement in symptoms within 48 to 72 hours, ciprofloxacin can be substituted unless the antibiogram suggests a more appropriate antibiotic. Nalidixic acid or nitrofurantoin can be used in children over the age of two years with suspected cystitis. Due to the high rate of antibiotic resistance, ampicillin, amoxicillin, cotrimoxazole, and cephalosporins are not recommended as the first-line choices for the empirical treatment of UTIs in children.