1. Background
It was February 2020 when the World Health Organization announced that coronavirus disease 2019 (COVID-19) had spread worldwide as a pandemic. Initially, it was assumed that the pediatric population was at low risk for COVID-19 infection; however, as time passed, mild to severe symptoms were reported in groups of children who experienced COVID-19 (1). According to reports, cardiac involvement might occur in children with COVID-19, even those who seem to be healthy (2-4).
Multiple organs can be affected by COVID-19 (2, 5-7); however, cardiovascular involvement might increase morbidity and mortality (1, 8, 9). Multisystem Inflammatory Syndrome of Children (MIS-C) is an example of a serious and even lethal form of cardiac involvement caused by this virus. These patients might present with myocardial injury, cardiac fibrosis, ventricular dysfunction, valvar regurgitation, arrhythmia, endothelial dysfunction, dysautonomia, coronary artery inflammation, aneurysm formation, thrombosis formation in cardiac chambers, and pulmonary vascular bed (9).
According to several studies, COVID-19 and MIS-C have different distributions based on age, gender, clinical manifestations, cardiac involvement, treatments, and outcomes (10). Given the disparities in the prevalence of each kind of the disease and their clinical characteristics and outcomes in different populations, the present survey aimed to study the clinical characteristics, laboratory features, and different manifestations of COVID-19 and MIS-C and their outcomes in a single tertiary center in the northwest of Iran. Moreover, this study included the data from 1 week and 3 months of follow-up of the patients to see how the clinical features would change over the time points that had not been previously evaluated. This is also the first report from northwest Iran describing different manifestations of COVID-19 and MIS-C in the pediatric population. Furthermore, to the best of our knowledge, this is the first pediatric COVID-19 study in Iran that consists of a comparison between pediatric intensive care unit (PICU) and non-PICU admitted patients.
2. Methods
This cohort retrospective study was performed in a single tertiary center in the northwest of Iran (Tabriz). This study included all symptomatic pediatric patients under 18 years who were admitted to Tabriz Children’s Hospital from March 2020 to June 2021. The patients either had a positive serologic test or real-time polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 or were critically ill, and their spiral chest computed tomography (CT) scan findings, and clinical and laboratory findings were in favor of COVID-19 infection. The MIS-C was defined according to the Royal College of Pediatrics and Child Health, United Kingdom definition (11). Then, they were divided into two groups, including COVID-19 and MIS-C. Although these patients underwent follow-ups at different intervals regarding their organ involvements, this survey included the data of two follow-up time points, namely 1 week and 3 months following admission. All the patients in the follow-up underwent a complete physical exam and more evaluations with echocardiography (ECG).
Medical records of the patients were reviewed by a trained researcher, and those who had incomplete records or survived but did not have follow-ups were excluded from the study. Demographic data, clinical presentation, underlying heart disease, ECG, echocardiographic findings, laboratory findings, pharmacological treatments, and outcomes of the patients were all extracted from their medical records.
Statistical analysis was performed using SPSS software (version 19). Frequency and mean values were used for qualitative and quantitative variables, respectively. In addition, the chi-square test, Fisher’s exact test, Mann-Whitney U test, independent sample t-test, and binary logistic regression model were used wherever eligible. The comparison of study variables (including age, gender, diagnosis, outcome, clinical presentation, different systems’ involvement, and laboratory findings) was assessed between PICU and non-PICU patients by univariate analysis, and those with a P-value less than 0.2 were entered into the logistic regression using the stepwise forward method to predict PICU admission. A P-value less than 0.05 was considered statistically significant.
3. Results
During this study (15 months), 151 patients met the inclusion criteria. The mean value of participants’ age was 5.34 ± 4.11 years. The youngest and the oldest patients were 5 days and 15 years, respectively. In this study, 78 (51.7%) and 73 (48.3%) patients had COVID-19 and MIS-C, respectively (Table 1). Although in the COVID-19 group, males and females were affected equally, MIS-C was more prevalent among male patients (P = 0.004). In the present study, 52 patients (34.4%) needed PICU admission, and there was no significant relationship between the need for PICU admission and diagnosis (P = 0.096). Regardless of diagnosis, the mean values of hospital stay and intensive care unit (ICU) stay were 8.3 ± 6.23, and 5.32 ± 2.73 days, respectively.
| Characteristics | COVID-19 (n = 78) | MIS-C (n = 73) | P-Value |
|---|---|---|---|
| Demographic Data | |||
| Age (y) | 5.44 ± 4.50 | 5.24 ± 3.67 | 0.759 b |
| Gender | 0.004 b | ||
| Male | 50 | 72.6 | |
| Female | 50 | 27.4 | |
| Length of hospital stay (day) | 9.24 ± 8.06 | 7.21 ± 2.94 | 0.510 c |
| Length of intensive care unit stay (day) | 6.87 ± 3.37 | 2.75 ± 2.04 | 0.378 c |
| Initial Presentation | |||
| Fever | < 0.001 b | ||
| Yes | 34 (43.6) | 64 (87.7) | |
| No | 44 (56.4) | 9 (12.3) | |
| Respiratory | < 0.001 b | ||
| Yes | 45 (57.7) | 14 (19.2) | |
| No | 33 (42.3) | 59 (80.8) | |
| Gastrointestinal | 0.628 b | ||
| Yes | 28 (35.9) | 29 (37.9) | |
| No | 50 (64.1) | 44 (60.3) | |
| Mucocutaneous | < 0.001 d | ||
| Yes | 3 (3.8) | 33 (45.2) | |
| No | 75 (96.2) | 40 (54.8) | |
| Neurologic | 0.444 d | ||
| Yes | 5 (6.4) | 2 (2.7) | |
| No | 73 (93.6) | 71 (97.3) | |
| Cardiologic | 0.650 d | ||
| Yes | 1 (1.3) | 2 (2.7) | |
| No | 77 (98.7) | 71 (97.3) | |
| Treatment | |||
| Intravenous immunoglobulin e | < 0.001 b | ||
| Yes | 5 (6.4) | 39 (53.4) | |
| No | 73 (93.6) | 34 (46.6) | |
| Steroid (dexamethasone or prednisolone) f | < 0.001 b | ||
| Yes | 22 (28.2) | 60 (82.1) | |
| No | 56 (71.8) | 13 (17.9) | |
| Antibiotic | < 0.001 b | ||
| Yes | 62 (79.4) | 35 (48) | |
| No | 16 (20.6) | 38 (52) | |
| Non-steroidal anti-inflammatory drugs | < 0.001 b | ||
| Yes | 8 (10.3) | 62 (85) | |
| No | 70 (89.7) | 11 (15) | |
| Favipiravir | < 0.001 d | ||
| Yes | 19 (24.4) | 3 (4.1) | |
| No | 59 (75.6) | 70 (95.9) | |
| Inotropes | 0.004 b | ||
| Yes | 6 (7.7) | 18 (24.7) | |
| No | 72 (92.3) | 55 (75.3) | |
| Diuretics | < 0.001 b | ||
| Yes | 7 (9) | 30 (41.1) | |
| No | 71 (91) | 43 (58.9) | |
| Pantoprazole | < 0.001 b | ||
| Yes | 17 (21.8) | 34 (46.57) | |
| No | 61 (78.2) | 39 (53.43) | |
Abbreviations: COVID-19, coronavirus disease 2019; MIS-C, Multisystem Inflammatory Syndrome of Children.
a Values are expressed as mean ± standard deviation, percentage or No. (%).
b P-value was calculated by the chi-square test.
c P-value was calculated by the Mann-Whitney U test due to length distributions.
d P-value was calculated by the chi-square Fisher’s exact test.
e In the COVID-19 group, intravenous immunoglobulin was prescribed for those with resistant shock status.
f For those with severe respiratory involvement following COVID-19 infection, dexamethasone was used. However, prednisolone was only used in the MIS-C group.
3.1. Clinical Presentations
The most common presentation was fever (64.9%). Respiratory, gastrointestinal, mucocutaneous, neurologic, and cardiovascular manifestations were observed in 39.1%, 37.7%, 36%, 4.6%, and 2% of the patients, respectively. Fever and mucocutaneous involvement were more prevalent in the MIS-C group; however, the respiratory system was more commonly involved in the COVID-19 group (Table 1).
3.2. Underlying Diseases
In this study, 21.8% of the patients (36.5% of PICU-admitted patients) had an underlying disease. Only 15 participants (9.9%) had congenital heart disease (CHD), and a surgical repair was previously carried out for about one-third of them.
3.3. Laboratory Findings Within the First 24 Hours of Admission
Approximately 85.3% of the patients in the COVID-19 group (the remaining patients were diagnosed according to clinical and laboratory findings in addition to positive pulmonary CT scans) and 32.8% of the patients in the MIS-C group had positive RT-PCR (P < 0.001).
The arterial blood gas analysis revealed that hypoxemia was more prevalent in COVID-19 patients. Nevertheless, decreased base excess was observed more commonly among the MIS-C patients (P < 0.001 and P < 0.015, respectively). However, pH and HCO3 did not differ significantly.
Table 2 shows the comparison of the laboratory results between the two groups (i.e., COVID-19 and MIS-C). Leukocytosis (P < 0.001), anemia (P = 0.034), thrombocytopenia (P < 0.001), and hypoalbuminemia (P = 0.054) were more common in the MIS-C group than in the COVID-19 group. Those with cardiac involvement had more leukocytosis (P = 0.025), lymphopenia (P < 0.001), and anemia (P = 0.004) and higher erythrocyte sedimentation rate (ESR) (P = 0.02) and C-reactive protein (CRP) level (P < 0.001). Among those for whom liver function test was checked, 75.6% of patients in the MIS-C group had hypoalbuminemia. There was no statistically significant relationship between the two groups prognosis according to the albumin level (Table 2). The present study showed that those who died had significantly more alanine transaminase (ALT) levels than those who survived (mean values: 175 ± 81.41 and 48.56 ± 42.66, respectively; P < 0.001).
| Variables | Diagnosis | P-Value | |
|---|---|---|---|
| MIS-C (n = 73) | COVID-19 (n = 78) | ||
| White blood cells | 11504.8 ± 6618.2 | 7693.6 ± 4925.2 | < 0.001 b |
| Normal | 43 (58.9) | 35 (44.9) | |
| Leukopenia | 5 (6.8) | 25 (32) | |
| Leukocytosis | 25 (34.3) | 18 (23.1) | |
| Anemia | 0.034 b | ||
| Yes | 41 (56.1) | 29 (37.2) | |
| No | 32 (43.9) | 49 (62.8) | |
| Platelet | < 0.001 b | ||
| Normal (150000 - 450000) | 29 (39.7) | 59 (75.6) | |
| Thrombocytopenia | 32 (43.8) | 13 (16.6) | |
| Thrombocytosis | 12 (16.5) | 6 (7.8) | |
| C-reactive protein | < 0.001 b | ||
| 0 | 6 (8.2) | 32 (41) | |
| 1+ | 7 (9.6) | 7 (9.0) | |
| 2+ | 32 (43.8) | 23 (29.5) | |
| 3+ | 28 (38.4) | 16 (20.5) | |
| Albumin | 0.054 b | ||
| Normal (3.8-5.1) | 10 (24.4) | 6 (54.5) | |
| Decreased | 31 (75.6) | 5 (45.5) | |
| Anti-SARS-CoV-2 IgG | 0.4 c | ||
| Negative | 6 (14.3) | 1 (33.3) | |
| Positive | 36 (85.7) | 2 (66.7) | |
| Anti-SARS-CoV-2 IgM | 0.34 c | ||
| Negative | 20 (47.6) | 1 (33.3) | |
| Positive | 22 (52.4) | 2 (66.7) | |
| Nasopharyngeal polymerase chain reaction | < 0.001 b | ||
| Negative | 22 (32.8) | 64 (85.3) | |
| Positive | 45 (67.2) | 11 (14.7) | |
Abbreviations: COVID-19, coronavirus disease 2019; MIS-C, Multisystem Inflammatory Syndrome of Children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IgG, immunoglobulin G; IgM, immunoglobulin M.
a Values are expressed as mean ± standard deviation or No. (%).
b P-value was calculated by the chi-square test.
c P-value was measured by Fisher’s exact test.
Those who had tachycardia that was disproportionate to the degree of fever underwent cardiac enzyme evaluation. Furthermore, 26 subjects underwent cardiac enzyme analysis (i.e., N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiac troponin I (CTNI)). Of these patients, 22 cases had elevated amounts of NT-proBNP; however, only one patient had an increased CTNI level. Although just one patient with elevated NT-proBNP level died, Fisher’s exact test failed to show a statistically significant relationship between NT-proBNP level and patient outcomes (P = 0.99). Although about half of the studied patients with elevated NT-proBNP levels on admission had no cardiac involvement on the first visit, evidence of cardiac involvement was observed by the end of the first week in one-third of the mentioned patients. However, by the end of the third month, no significant cardiac involvement was observed in the mentioned population.
3.4. Chest X-ray and High-resolution Computed Tomography of Lung Findings
Abnormal chest X-ray (CXR) findings were more prevalent in the patients who were diagnosed with COVID-19 (P = 0.020). All kinds of lung infiltration patterns were observed in 66.2% and 49.2% of COVID-19 and MIS-C patients’ CXR, respectively (P = 0.048). Among those who underwent more detailed evaluation by lung CT, there was no statistically significant difference between these two groups regarding lung infiltration (P = 0.189); however, atelectasis and pleural effusion were more prevalent in the MIS-C patients (P = 0.009 and P = 0.005, respectively).
3.5. ECG Findings
Sinus tachycardia was the only arrhythmia observed in the present study that was completely resolved in the follow-up. Both on admission and in follow-ups, P-wave, PR interval, QRS durations, voltage, QTc, and T-wave were within the normal range for the age of all the participants except the patients with hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM).
3.6. Echocardiographic Findings
Only one patient had HCM, and another had DCM. Normal left ventricular (LV) ejection fractions were reported in 65.3% of the MIS-C group and 88.9% of the COVID-19 group on admission and totally in 90% of them in the third-month follow-up. In addition, right ventricular (RV) function was normal for all patients except one patient, for whom it was retained to the normal range in the follow-up. The most common valves involved were mitral and tricuspid valves. Moreover, there were 31 children with mild to moderate mitral regurgitation (MR) in the first visit; however, no MR was observed by the end of the third month. Furthermore, 32 subjects had mild to moderate tricuspid regurgitation (TR) on admission. Additionally, in the third month of follow-up, nine patients had mild TR. The aortic valve was spared for all patients. Pulmonary insufficiency was noticed in one patient on the first visit, two patients after 1 week, and five children 3 months later. Coronary artery dilation was observed mostly in the left main coronary artery (LMCA). Four patients had mild LMCA dilation on admission. Retaining to the normal size was attained in three of them after the first week and in the remaining patients in the third-month follow-up. Brightness in the course of the major coronary arteries was mostly observed in the right coronary artery. No coronary involvement was noticed after the third month. There were three patients who developed mild pericardial effusion after 1 week. However, in the third-month follow-up, no pericardial effusion was detected by ECG.
3.7. Cardiac Involvement
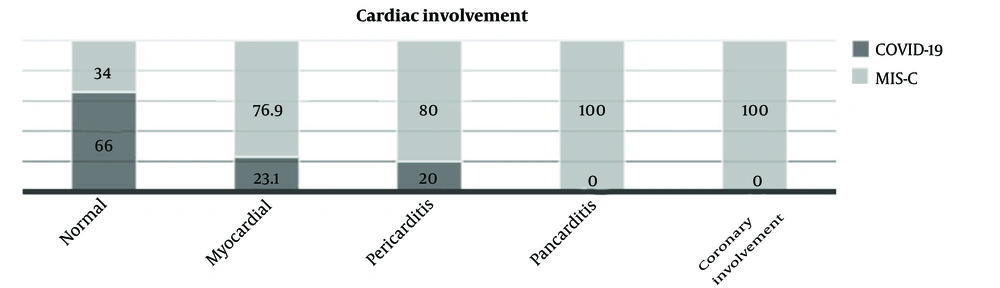
Regardless of the patient diagnosis, 41 patients (27.2%) had evidence of cardiac involvement. Endocarditis was diagnosed for none of the patients. Pericarditis, myocarditis, and coronary artery involvement were observed in 7.3%, 8.6%, and 11.3% of the patients, respectively. Figure 1 depicts the prevalence of different types of cardiac involvement in COVID-19 and MIS-C groups. Table 3 shows correlated factors for cardiac involvement in pediatric patients with COVID-19 and MIS-C.
| Variables | Cardiac Involvement | P-Value b | |
|---|---|---|---|
| No | Yes | ||
| Gender | 0.012 | ||
| Male | 60 (55.5) | 33 (76.7) | |
| Female | 48 (44.5) | 10 (23.3) | |
| Diagnosis | < 0.001 | ||
| COVID-19 | 71 (65.7) | 6 (13.9) | |
| MIS-C | 37 (34.3) | 37 (86.1) | |
| Intensive care unit stay | < 0.001 | ||
| No | 80 (74.0) | 18 (41.8) | |
| Yes | 28 (26.0) | 25 (58.2) | |
| Chest X-ray | 0.651 | ||
| Normal | 37 (39.4) | 17 (43.6) | |
| Abnormal | 57 (60.6) | 22 (56.4) | |
| White blood cells | 0.025 | ||
| Normal | 54 (50.0) | 22 (51.1) | |
| Leukopenia | 27 (25.0) | 3 (7.0) | |
| Leukocytosis | 27 (25.0) | 18 (41.9) | |
| Platelet | 0.164 | ||
| Normal | 66 (61.1) | 21 (48.8) | |
| Thrombocytopenia | 28 (25.9) | 18 (41.8) | |
| Thrombocytosis | 14 (13.0) | 4 (9.4) | |
| C-reactive protein | 0.044 | ||
| 0 | 33 (30.5) | 5 (11.6) | |
| 1+ | 11 (10.1) | 3 (7.0) | |
| 2+ | 33 (30.5) | 21 (48.8) | |
| 3+ | 31 (28.0) | 14 (32.6) | |
| COVID-19 IgG | - | ||
| Negative | 6 (28.6) | 1 (4.2) | |
| Positive | 15 (71.4) | 23 (95.8) | |
| COVID-19 IgM | 0.005 | ||
| Negative | 13 (61.9) | 8 (33.3) | |
| Positive | 8 (38.1) | 16 (66.7) | |
| COVID-19 polymerase chain reaction | < 0.001 | ||
| Negative | 71 (70.3) | 13 (33.3) | |
| Positive | 30 (29.7) | 26 (66.7) | |
Abbreviations: COVID-19, coronavirus disease 2019; MIS-C, Multisystem Inflammatory Syndrome of Children; IgG, immunoglobulin G; IgM, immunoglobulin M.
a Values are expressed as No. (%).
b P-value was calculated by the chi-square test.
3.8. PICU Admission
Approximately 34.4% of the patients (41.1% of MIS-C and 28.2% of COVID-19 patients) were admitted to the PICU. Table 4 shows the comparison between the PICU and non-PICU admitted patients with MIS-C or COVID-19. According to the binary logistic regression stepwise forward method, those who were in the neonates’ group and had cardiac involvement or underlying diseases were at a higher risk for ICU admission (P < 0.001, Table 5).
| Variable | Non-PICU | PICU | P-Value |
|---|---|---|---|
| Gender | 0.993 b | ||
| Male | 61 (61.62) | 32 (61.54) | |
| Female | 38 (38.38) | 20 (38.46) | |
| Age (y) | 5.52 ± 4.26 | 5.24 ± 4.23 | 0.70 c |
| Diagnosis | 0.1 b | ||
| COVID-19 | 56 (56.57) | 22 (42.31) | |
| MIS-C | 43 (43.43) | 30 (57.69) | |
| Outcome | 0.001 d | ||
| Discharged | 99 (100) | 46 (88.46) | |
| Died | 0 (0) | 6 (11.54) | |
| Underlying disease | 0.002 c | ||
| Yes | 14 (14.14) | 19 (36.54) | |
| No | 85 (85.86) | 33 (63.46) | |
| Fever | 0.53 b | ||
| Yes | 66 (66.67) | 32 (61.54) | |
| No | 33 (33.33) | 20 (38.46) | |
| Respiratory | 0.64 b | ||
| Yes | 40 (40.4) | 19 (36.54) | |
| No | 59 (59.6) | 33 (63.46) | |
| Gastrointestinal | 0.058 b | ||
| Yes | 32 (32.32) | 25 (48.08) | |
| No | 67 (67.68) | 27 (51.92) | |
| Mucocutaneous | 0.81 b | ||
| Yes | 23 (23.23) | 13 (25) | |
| No | 76 (76.77) | 39 (75) | |
| Neurologic | 0.23 b | ||
| Yes | 3 (3.03) | 4 (7.69) | |
| No | 96 (96.97) | 48 (92.31) | |
| Cardiologic | 0.04 d | ||
| Yes | 0 (0) | 3 (5.77) | |
| No | 99 (100) | 49 (94.23) | |
| Inotropic agent | < 0.001 d | ||
| Yes | 3 (2.04) | 11 (19.61) | |
| No | 96 (97.96) | 41 (80.39) | |
| Diuretic | < 0.001 c | ||
| Yes | 4 (4.08) | 13 (25.49) | |
| No | 94 (95.92) | 39 (74.51) | |
| Intravenous immunoglobulin | 0.38 c | ||
| Yes | 26 (26.53) | 17 (33.33) | |
| No | 73 (73.47) | 35 (66.67) | |
| Antibiotic | < 0.001 c | ||
| Yes | 54 (54.08) | 44 (84.31) | |
| No | 45 (45.92) | 8 (15.69) | |
| Acetylsalicylic acid | 0.52 c | ||
| Yes | 37 (37.76) | 22 (43.14) | |
| No | 62 (62.24) | 30 (56.86) | |
| Anti-SARS-CoV-2 IgG | 0.41 c | ||
| Negative | 3 (27.7) | 4 (11.76) | |
| Positive | 8 (72.72) | 30 (88.23) | |
| Anti-SARS-CoV-2 IgM | 0.46 c | ||
| Negative | 3 (37.5) | 18 (48.64) | |
| Positive | 5 (62.5) | 19 (51.35) | |
| Nasopharyngeal polymerase chain reaction | 0.32 b | ||
| Negative | 60 (80) | 18 (30.50) | |
| Positive | 15 (20) | 41 (69.5) | |
| White blood cells | 9087.14 ± 5379.21 | 10423.78 ± 7250.84 | 0.206 c |
| Hemoglobin | 11.62 ± 1.84 | 11.87 ± 2.2 | 0.469 c |
| Platelet | 274309.28 ± 192398.04 | 244980.39 ± 168984.2 | 0.360 c |
Abbreviations: PICU, pediatric intensive care unit; COVID-19, coronavirus disease 2019; MIS-C, Multisystem Inflammatory Syndrome of Children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IgG, immunoglobulin G; IgM, immunoglobulin M.
a Values are expressed as No. (%) or mean ± standard deviation.
b P-value was measured by chi-square test.
c P-value was measured by independent sample t-test.
d P-value was measured by Fisher’s exact test.
| Factors a | Odds Ratio | 95% Confidence Interval for Odds Ratio | P-Value b | |
|---|---|---|---|---|
| Gender (female/male) | 1.048 | 0.38 | 2.88 | 0.928 |
| Age (neonate/others) | 33.11 | 4.28 | 256.17 | < 0.001 |
| Body surface area | 1.25 | 0.66 | 0.2.36 | 0.494 |
| Blood pressure (hypotension/normal) | 2.55 | 0.75 | 8.68 | 0.135 |
| Temperature (fever/normal) | 1.54 | 0.57 | 4.13 | 0.398 |
| Respiratory rate (tachypnea/normal) | 1.50 | 0.56 | 3.94 | 0.426 |
| Underlying diseases (yes/no) | 6.94 | 2.24 | 21.47 | < 0.001 |
| White blood cells (abnormal/normal) | 1.34 | 0.80 | 2.26 | 0.210 |
| Hypoxia (hypoxia/normal) | 0.82 | 0.30 | 2.23 | 0.70 |
| Cardiac involvement (yes/no) | 8.75 | 2.84 | 27.0 | < 0.001 |
| Gastrointestinal involvement (yes/no) | 1.43 | 0.57 | 3.62 | 0.451 |
| Neurologic involvement (yes/no) c | 2.06 | 0.26 | 16.32 | 0.495 |
| Mucocutaneous involvement (abnormal/normal) c | 0.73 | 0.17 | 3.06 | 0.663 |
a Some variables, such as diagnosis, inotropic agent, diuretic, and antibiotic, were not entered into the logistic regression analysis despite having P-values < 0.2 because they had co-linearity. Outcome (discharged/died) cannot be a predictor for PICU admission; therefore, it was not included in the logistic regression analysis.
b P-value was calculated by the binary logistic regression model.
c This variable was entered into the logistic regression analysis due to the clinical importance despite having a P-value > 0.2.
3.9. Treatments and Outcomes
Antibiotics, steroids, intravenous immunoglobulin (IVIG), and inotropic agents were the most common medications used by patients. Table 1 shows the patients’ treatments. About 25% of the PICU-admitted patients were intubated, 46% of whom expired. About 96.1% of the patients survived and had improvement. Among them, there was no abnormality in the third-month follow-up in the neurologic, respiratory, and gastrointestinal systems. Furthermore, for most patients with cardiovascular involvement, normal ventricular and valvar function and coronary arteries were obtained in the third-month follow-up. Moreover, among those who died, all the patients had refractory hypotension, two patients had respiratory failure, and one case presented super-refractory status epilepticus. Prolonged capillary refill time was associated with a higher mortality rate (P = 0.05). Aspirin with an anti-inflammatory dose was not prescribed for patients with thrombocytopenia. All the patients who received acetylsalicylic acid (ASA) survived; however, only 92.34% of those for whom ASA was not prescribed were discharged from the hospital (P = 0.043). In the MIS-C group, the mortality rate was 11.11%; nevertheless, the mortality rate was 2.56% in the COVID-19 group (P = 0.049).
4. Discussion
This retrospective study from northwest Iran described and compared pediatric patients with COVID-19 and MIS-C regarding their clinical characteristics, laboratory findings, and outcomes of the disease. One of the main advantages of this study was the focus on the differences between the outcomes and characteristics of patients admitted to the PICU and those who were not and considering three time-points in follow-up duration to fully assess the results of patients.
In the present study, the prevalence of COVID-19 was equal in both genders; however, MIS-C was more common in males, which is consistent with the results of studies from Latin America and the United Kingdom (12, 13).
The most common symptoms in the current study were fever and respiratory symptoms. A study of 59 pediatrics with COVID-19 from Iran reported respiratory distress and dyspnea as the most common symptoms (14). Similarly, a systematic review of 7780 children revealed that fever and cough were the most common clinical presentations (15). Central nervous system involvement could be observed in COVID-19 (6). In the present study, two patients presented with encephalitis and status epilepticus.
In this study, six patients died, all of whom had an underlying disease, and were admitted to the PICU. At the beginning of this pandemic, the mortality rate in the PICU was reported to be up to 50%, which was mostly observed in patients with underlying diseases (1). In a study from Norway, all COVID-19 patients under 20 years of age survived (16). However, in a Brazilian report, the mortality rate was 5.6% among 682 patients (17). In the current studied patients, having delayed capillary refill time and raised ALT levels were associated with a higher mortality rate. A study from England concluded that the death hazard in diabetic patients was two times higher than in non-diabetic ones (18). In the present study, refractory hypotension was the main cause of mortality; therefore, the approach to hypotension and its treatment differs from conventional therapies (19).
Cardiovascular involvement was observed in 27.2% of the participants. Myocarditis, pericarditis, and coronary arteries inflammation were observed in 8.6%, 7.3%, and 11.3% of the subjects, respectively. Patients with MIS-C had significantly more cardiac involvement either on admission or in the follow-up. This finding is in line with the results of an Iranian case-series study, suggesting that cardiac consultation for MIS-C patients would improve the chance of survival (20). The results of a Brazilian study support the present study’s findings (21). No coronary abnormality was observed in the third-month follow-up; however, eight patients had mild LV dysfunction, and one who was readmitted with MIS-C had moderate LV dysfunction. Such a transient coronary dilation in the acute phase of the disease is thought to be the responsible compensatory mechanism to overcome the increased myocardial oxygen demand due to myocarditis, endothelial malfunction, local hypoxia, and fever (22). Similarly, in a Brazilian investigation, the most common echocardiographic finding was coronary dilation. Higher D-dimer levels had an association with ventricular dysfunction and coronary dilation (21). In another study on 28 patients with MIS-C and 20 healthy individuals, LV systolic function was retained to the normal range in subacute phase follow-up; nevertheless, LV diastolic dysfunction and RV dysfunction persisted (23). Another investigation marked LV and RV dysfunction and TR > grade 1 as predictors of fatal COVID-19 (24).
Arrhythmia can be observed in the patients; however, sinus tachycardia has been reported more frequently and might be due to some factors, such as fever, increase in insensible water loss, and hypoxia (25). In the present study, sinus tachycardia was the only arrhythmia observed without linkage with worse outcomes and was resolved by conservative management. A study demonstrated that patients with ST elevation had a poor prognosis, as ST elevation is an indicator of myocardial ischemia as a result of thrombosis formation in the coronary vascular bed (26). This highlights the importance of undergoing cardiac evaluation by ECG for all the patients on admission and repeating the evaluation in follow-ups.
Leukocytosis, lymphopenia, anemia, elevated ESR, and CRP level were all risk factors that make patients prone to cardiovascular system involvement. A Lebanese study revealed that with more leukocytosis, the probability of severe COVID-19 becomes greater (4). Higher CRP and D-dime levels were associated with an increase in disease severity (27). In addition, an inverse association between the degree of lymphocyte count with disease severity and poorer outcome has been shown elsewhere (28, 29). Consistent with the present study’s findings, mean platelet volume was not associated with disease severity (30).
Abnormal liver tests in children with COVID-19 are frequent, and hypoalbuminemia can be considered a poor prognostic factor in addition to high lactate dehydrogenase and CRP (5). Although 75.6% of patients in the MIS-C group had hypoalbuminemia in the current study, there was no statistically significant relationship between the two groups’ prognosis regarding their albumin level (Table 2). Increased liver enzymes could be noticed in COVID-19 patients, which could be due to the direct insult of the virus or the severity of the disease (5).
In the present study, 21.8% of patients (36.5% of PICU-admitted patients) had underlying diseases, and 15 participants (9.9%) had CHD. There was no association between having underlying diseases and CHD with patient outcomes. A meta-analysis marked CHD as a risk factor for ICU admission but not death (28). An Indian multicenter survey also revealed that an underlying cardiac disease would increase mortality (31). British Congenital Cardiac Association listed the number of patients with CHD prone to developing severe forms of the disease, including cyanotic CHD with oxygen saturation < 85%, pulmonary hypertension, and patients with cardiomyopathy who are under medical management (32). This discrepancy could be explained by parents’ concerns because they followed preventive protocols more precisely, and almost all these patients were visited by their cardiologist within the first 24 hours of symptom appearance. Moreover, in their country, the follow-up system of such cardiac pediatric patients is highly advanced. Therefore, the early diagnosis and proper management of such patients played a key role in better outcomes.
Cardiac involvement and cardiac biomarkers elevation in MIS-C patients differ in different populations and have even been estimated in up to 80% of the patients (33). A high level of NT-proBNP in acute COVID-19 infection has been reported (7, 34). A Turkish study revealed that proBNP > 282 ng/L has a sensitivity of 100% and specificity of 93% for MIS-C development prediction; however, CTNI has less but still noticeable sensitivity and specificity; therefore, they can be used for the early diagnosis of cardiac involvement and outcome anticipation in COVID-19 patients (35). In the present study, 22/26 patients had raised amounts of NT-proBNP; nevertheless, only one case had a high CTNI level. Although about half of the studied patients with elevated NT-proBNP levels on admission had no cardiac involvement on the first visit, evidence of cardiac involvement was observed in the first-week follow-up in one-third of them. All these patients had normal echocardiographic findings in the third-month follow-up. This is probably because bimolecular changes in the myocytes almost always proceed with the gross changes, which can be detected by ECG; therefore, it can be used as a predictive measurement for cardiac involvement in pediatric patients with COVID-19 (36). However, further investigation is needed in this area.
No specific treatment has been suggested for this disease, and the mainstay of treatment is supportive management which can be even life-saving provided that it starts at the proper time. After antibiotics, the most common treatments of the studied participants were steroids, IVIG, and ionotropic agents. Those who received ASA had a lower mortality rate, and this finding might be attributed to the dosage of the drug that was used. All these patients received a high dosage of ASA that has an anti-inflammatory effect. Since hyper-inflammation and cytokine storm are among the proposed mechanism responsible for the pathogenesis of this viral disease, any strategy that can subside the inflammation might have an important role in improving the symptoms and decreasing mortality (27). The protective effect of ASA against severe forms of COVID-19 was shown in previous studies (4).
The major limitation of the present study was that the cardiac biomarkers and the other blood tests were not checked in the follow-up.
4.1. Conclusions
The early diagnosis, proper management, and protocolized follow-up of pediatric patients with COVID-19 or MIS-C can prevent adverse events and be life-saving.