1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the ongoing pandemic. Published reports indicate that respiratory symptoms, such as pneumonia and inflammatory conditions, are common in this disease (1-3). As the pandemic has spread, numerous cases of patients suffering from thrombotic events associated with coronavirus disease 2019 (COVID-19) have been reported. The incidence of both arterial and venous damage in these patients was higher than in non-affected individuals. Therefore, the reported risk of venous and arterial thromboembolism in patients with COVID-19 is 16% and 11.1%, respectively (4). In addition, several autopsy studies have shown the presence of microthrombi in various organs of the body, including the kidney, heart, skin, and lungs, in COVID-19 patients with microangiopathy (5, 6).
Antiphospholipid (aPL) antibody syndrome is an autoimmune disorder characterized by the presence of aPL antibodies and clinical manifestations, including arterial/venous thrombotic events. Antiphospholipid has long been recognized as one of the contributing factors in the development of hypercoagulability and thrombosis (7). In some patients with COVID-19, the disease might interfere with the function of coagulation modifiers and lead to conditions of increased coagulability, which are associated with a poor prognosis according to available information (8-10). Common criteria for the diagnosis of aPL syndrome include the presence of lupus anticoagulant (LAC), anticardiolipin (aCL) antibodies, and anti-β2 glycoprotein I (aβ2GPI) (11).
Several studies have examined aPL antibodies in patients with COVID-19 (7, 12); however, few studies have evaluated affected children.
2. Objectives
The current study aimed to evaluate the levels of aPL antibodies in children with and without COVID-19. If an association is observed between severe COVID-19 and the occurrence of serious side effects and a high level of aPL antibodies, it might be possible to predict the side effects and take precautionary measures by evaluating this factor in infants.
3. Methods
3.1. Study Design
This descriptive-analytical cross-sectional study was conducted on patients under 16 years of age, both with and without COVID-19, who were admitted to Ali Asghar hospital within December 2021 and February 2022. The study was conducted after obtaining approval from the Biomedical Studies Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (code: IR.IUMS.FMD.REC.1400.508). The participants and their parents were informed that participation in the study was voluntary and were required to grant parental consent after receiving a detailed explanation of the study procedures. Based on previous studies by Xiao et al. (7) and Zuo et al. (13), a sample size of 33 patients in each group (99 patients in total) was determined.
3.2. Participants and Data Collection
The patients in the case group were evaluated by a pediatrician, based on the coronavirus disease 2019 (COVID-19) treatment guidelines, for the presence of clinical symptoms and were classified into non-severe and severe groups. Infants with lower respiratory tract infections during clinical assessment or imaging (LRTIs) and oxygen saturation (SpO2) ≥ 94% on room air were classified as non-severe. Those with SpO2 < 94% on room air, arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio < 300 mm Hg, lung infiltrates > 50%, or who were tachypneic were classified as severe. Solid-phase enzyme immunoassay was used to measure blood aPL antibody levels in the case group.
Sampling was performed on the first day of admission. All infants in the case group were hospitalized, and none had thromboembolism. The unaffected group consisted of children hospitalized for elective surgery. To ensure that patients in the control group were not infected with COVID-19, they underwent polymerase chain reaction (PCR) testing prior to surgery. Those who tested negative were included in the study. Patient information was recorded by the investigator using a checklist that included demographic information (age), clinical findings, and laboratory test information (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], D-dimer, aPL antibody, aCL antibody, and aβ2GPI). Solid-phase enzyme-linked immunosorbent assay (ELISA) was used to measure aCL antibody and aPL antibody. MPL/mL and GPL/mL units were used as the measurement units for aCL antibody and MPL.
3.3. Statistical Analysis
Kolmogorov-Smirnov and Shapiro-Wilk tests were used to ensure that the data were normally distributed. The data were expressed as mean ± standard deviation (M ± SD). The sample size was estimated with 80% power and a type 1 error of 0.05 to detect a moderate effect size. Data analysis included the use of statistical tests, including independent t-test, one-way analysis of variance (ANOVA), and Kruskal-Wallis analysis. In addition, multiple logistic regression analysis was used to assess the adjusted association of the measured variables with the severity of COVID-19. The dependent variable was the severity of COVID-19 (two groups), and covariates were variables with a significant level of less than 0.3. The SPSS software package (version 22.0, SPSS Inc, Chicago, IL, USA) was used for data analysis, and a significance level of 0.05 was applied.
4. Results
In this study, 99 patients were examined and divided into three groups: Control (without COVID-19), non-severe, and severe. The mean age of the patients was 3.83 ± 4.21 years (with a minimum and maximum age of 1 and 15 years, respectively), and there was no significant difference between the three groups (P > 0.05). Most patients (71.21%) had symptoms of pulmonary involvement, and no significant difference was observed between the two non-severe and severe groups (P > 0.05).
The mean (SD) of CRP and D-dimer levels were significantly higher in the severe group (P < 0.05) (Table 1).
| Variables | Non-Severe, Mean ± SD | Severe, Mean ± SD | P-Value a |
|---|---|---|---|
| ESR | 28.26 ± 29.26 | 46.33 ± 45.99 | 0.061 |
| CRP | 26.40 ± 29.80 | 59.70 ± 59.16 | 0.005 |
| D-dimer | 550.76 ± 646.58 | 1250.15 ± 1129.65 | 0.003 |
Abbreviations: ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a Independent t-test
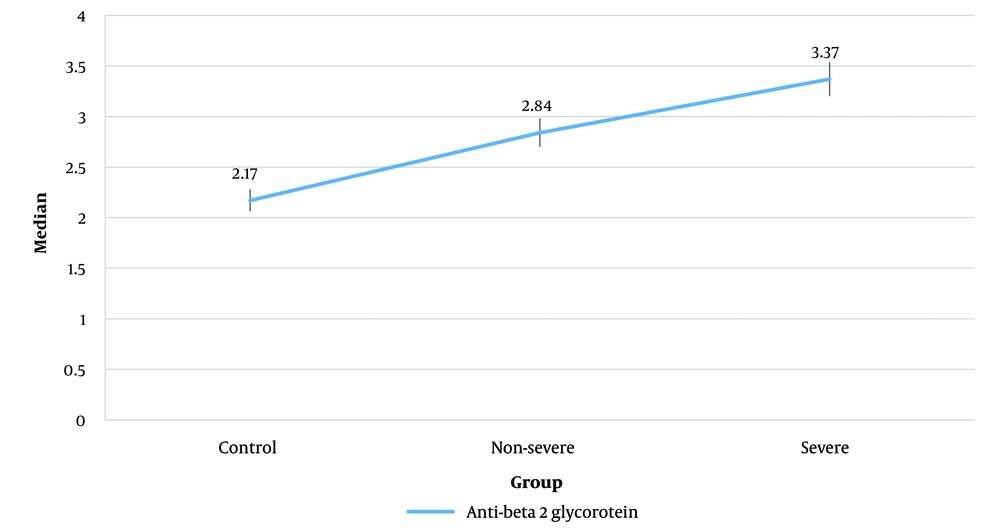
To compare the mean (SD) of aCL antibody and aPL antibody levels among the three groups, a one-way ANOVA test was performed. The results showed that there was no significant difference among the three groups (P > 0.05, see Table 2). Due to the lack of normal distribution of aβ2GPI, the Kruskal-Wallis test was used, which indicated no significant differences among the three groups (P > 0.05) (Figure 1).
| Variables | Control, Mean ± SD | Non-Severe, Mean ± SD | Severe, Mean ± SD | P-Value a |
|---|---|---|---|---|
| aCL antibody | 5.78 ± 5.69 (1 - 23) | 5.47 ± 3.57 (1 - 12) | 6.49 ± 5.69 (1 - 23) | 0.704 |
| aPL antibody | 3.44 ± 2.60 (1 - 11) | 4.01 ± 3.41 (0 - 11) | 4.69 ± 3.29 (0 - 12) | 0.267 |
Abbreviations: aCL, anticardiolipin; aPL, antiphospholipid.
a One-way analysis of variance (ANOVA)
The Pearson correlation coefficient test was used to investigate the relationship between aPL antibody and aCL antibody levels with age. The results revealed that there was no significant relationship between age and aPL antibody (r = -0.095, P > 0.05). However, a weak inverse relationship was observed between age and aCL antibody levels (r = -0.0281, P = 0.022). In other words, the R-value indicates the degree of relationship between two continuous variables.
The reported coefficient of correlation was calculated by controlling for group variables. No significant correlation was observed between age and aPL antibody (P > 0.05). The only significant and direct relationship was observed between aCL antibody and D-dimer levels (P = 0.006) (Table 3).
| Variables | aCL Antibody | aPL Antibody |
|---|---|---|
| Age | ||
| Pearson correlation coefficient | -0.085 | -0.272 |
| P-value | 0.502 | 0.028 |
| ESR | ||
| Pearson correlation coefficient | 0.168 | 0.030 |
| P-value | 0.178 | 0.811 |
| CRP | ||
| Pearson correlation coefficient | 0.051 | 0.163 |
| P-value | 0.684 | 0.190 |
| D-dimer | ||
| Pearson correlation coefficient | 0.334 | 0.183 |
| P-value | 0.006 | 0.141 |
Abbreviations: aCL, anticardiolipin; aPL, antiphospholipid; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
The results of multiple logistic regression are shown in Table 4. The association of CRP and D-dimer with COVID-19 severity remained significant with dilution after adjustment. There was also no association between aPL antibody and aCL antibody with disease severity after adjustment.
| Variables | B | P-Value | OR | Lower OR95% | Upper OR95% |
|---|---|---|---|---|---|
| Constant | -1.389 | 0.048 | 0.249 | - | - |
| ESR | 0.000 | 0.984 | 1.00 | 0.983 | 1.017 |
| Age | -0.095 | 0.269 | 0.909 | 0.767 | 1.077 |
| CRP | 0.019 | 0.038 | 1.019 | 1.001 | 1.038 |
| D-dimer | 0.001 | 0.021 | 1.001 | 1.000 | 1.002 |
| aCL antibody | 0.044 | 0.456 | 1.045 | 0.931 | 1.174 |
| aPL antibody | -0.007 | 0.944 | 0.993 | 0.814 | 1.212 |
Abbreviations: OR, odds ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; aCL, anticardiolipin; aPL, antiphospholipid.
5. Discussion
Vascular thrombosis is a serious and common complication in severely ill patients with COVID-19. Children are less likely to develop this complication than adults; however, they are at risk for thrombosis during acute infection (14). This study aimed to compare the levels of aPL antibodies between children with COVID-19 (moderate and severe) and unaffected children. Although the levels of CRP and D-dimer were significantly higher in the severe disease group, there was no significant difference in the mean levels of aCL antibody, aPL antibody, and aβ2GPI among the three groups.
Recent evidence has shown a significant increase in aPL immunoglobulin A (IgA) associated with severe COVID-19. It is likely that the effects of SARS-CoV-2 on the respiratory system trigger a strong IgA-based immune response. However, there was no significant correlation with immunoglobulin G (IgG) (15). The aforementioned findings are in contrast to the present study’s results.
Pineton De Chambrun et al. conducted a retrospective study to investigate the association between antiphospholipid antibodies (APLA) and hypercoagulability in patients with COVID-19. Twenty-five patients with SARS-CoV-2 treated in an intensive care unit were included in the study. The results showed that APLA is not necessarily associated with thrombosis, especially when it is not permanent (16).
In recent studies, approximately one-third of patients with severe disease developed thrombosis (17), which was not associated with D-dimer levels (18, 19). These thrombi might be explained by the increase in total IgA and IgA of aPL, which have been significantly associated with severe disease according to studies such as the present study. However, this association was not observed for total IgG or IgG of aPL antibodies (15). Although the association between high aPL antibody levels and severe COVID-19 has been reported, elevated total IgA levels, along with IgA of aPL when comparing mild and severe COVID-19, suggest that there is no association between the immune response and hypercoagulability or the initiation of aPL syndrome (20).
Xiao et al. reported in a study that the amount of aPL antibody in patients with severe COVID-19 is unknown (7), which is consistent with the current study’s findings. In some patients, a transient increase in aPL antibody might be associated with thrombotic complications. It is important to note that although these antibodies disappear within a few weeks in some patients, COVID-19 might cause an antiphospholipid syndrome (APS)-like syndrome in other genetically predisposed patients. It would be useful to have long-term follow-up of patients with COVID-19 who are aPL antibody positive.
Benjamin et al. demonstrated that anti-phosphatidylserine/prothrombin antibody (aPS/PT) IgG titers were significantly higher in the neurologic group of COVID-19 than in both control groups (P < 0.001). Moderate and high aPS/PT IgG titers were observed in two out of three patients (68%) with acute disseminated encephalomyelitis (ADEM). The aPS/PT IgG titers were negatively correlated with oxygen demand (P = 0.041) and associated with venous thromboembolism (P = 0.044). In contrast, IgA of aCL antibody (P < 0.001) and IgG (P < 0.001) were associated in the non-neurologic control groups hospitalized with COVID-19, compared to the other groups and were positively correlated with D-dimer and creatinine, confirming the present study’s results. However, the association with FiO2 was negative (21).
A high frequency (58%) of both aPL antibodies in patients with severe and critical COVID-19 was reported by Amezcua-Guerra et al. In this study, these aPL antibodies appear to be associated with a hyperinflammatory state characterized by high levels of ferritin, CRP, and interleukin 6. There might also be an association with pulmonary thromboembolism. The study showed that various aPL antibodies can occur transiently during acute infection, thrombosis, or inflammation, and it should not be assumed that a patient with coagulopathy associated with COVID-19 and aPL antibodies has a poor prognosis (22). However, this study showed that cardiovascular complications were significantly more common in the group with high CRP and D-dimer.
Tang et al. studied high levels of D-dimer products and fibrin degradation to determine patients’ prognosis and risk of thrombosis (10). Zhang et al. described three cases of thrombosis associated with aPL antibody in conjunction with aCL antibody and aβ2GPI (23), confirming the results of the present study. The current study also demonstrated a significant and direct association between aCL antibody and D-dimer. During the recent COVID-19 outbreak in Mulhouse, France, 25 cases (46%) were positive for LAC; nevertheless, aCL antibody or aβ2GPI were detected in only 5 of 50 patients examined (11%, 3 cases associated with LAC). It was identified from IgG and immunoglobulin M (IgM) (24). Acute infections are sometimes associated with transient LAC and usually do not require anticoagulant therapy (25). Therefore, the diagnosis of LAC, with or without aCL antibody or aβ2GPI, underscores the importance of early anticoagulation therapy in these critically ill patients who have numerous risk factors for thrombosis.
Neijmann et al. conducted a study to investigate the prevalence of aPL antibody in COVID-19 patients and its association with clinical outcomes. The aforementioned study showed that aPL antibody was present in 17.8% of COVID-19 patients and was associated with a higher risk of thrombosis and mortality (26). This finding is consistent with the view that aPL antibodies might play an important role in COVID-19 thrombosis, as suggested by Gil-Etayo et al. (27). A study by Shah et al. further investigated the association between aPL antibody and thrombosis in COVID-19 patients with and without vitamin D deficiency. The study concluded that aPL antibody and vitamin D deficiency might contribute to the risk of thrombosis in COVID-19 patients (28). However, the role of aPL antibody in COVID-19 remains controversial. Foret et al. performed a systematic review of studies investigating aPL antibody in COVID-19 patients and obtained conflicting results (12). They concluded that aPL antibody might be present in COVID-19 patients; nonetheless, it is unclear whether they play a causal role in thrombosis or are merely bystanders. Stelzer et al. also investigated the role of aPL antibody in COVID-19 and concluded that aPL antibody might be associated with severe disease and thrombosis in some patients (29). However, further studies are needed to clarify their role.
5.1. Conclusions
The elevated levels of CRP and D-dimer in children with COVID-19 are associated with more severe disease. However, there was no significant relationship between disease severity and the levels of aCL antibody, aPL antibody, and aβ2GPI in children.