1. Background
Carbapenems are the most broad-spectrum beta-lactam antibiotics against gram-negative bacteria. Most beta-lactamases cannot break down these antibiotics. As a result, medications in this class are employed as the final line of defense against gram-negative bacteria resistant to other antibiotics (1). Resistance to several antibiotics has been reported in the Enterobacteriaceae family in recent decades, forcing clinicians to administer carbapenems as a last resort. Carbapenem resistance was not discovered in the United States until the year 2000. Unlike methicillin resistance in Staphylococcus aureus species, which occurs and is caused by a single mechanism, carbapenem resistance in Enterobacteriaceae occurs in several members of this family and is caused by various distinct mechanisms (2, 3). Among the Enterobacteriaceae family, Klebsiella pneumoniae and Escherichia coli species produce more carbapenemase enzymes. Carbapenem-resistant Enterobacteriaceae (CRE) show different resistance characteristics that depend entirely on genetic elements and the type of carbapenemase they produce. The carbapenemase-producing Enterobacteriaceae family has developed resistance that can easily be transmitted between them and other species. The most dominant type of carbapenemase created worldwide is related to the bacterium K. pneumoniae, which is associated with the blaKPC gene. Other identified carbapenemases related to the epidemic of antibiotic resistance in Enterobacteriaceae include Verona integron-encoded metallo-ß-lactamase (VIM), New Delhi metallo-b-lactamase (NDM), imipenemase (IMP), and oxacillinase- 48-type (OXA-48) (4, 5). Due to the importance of patient-to-patient transmission of resistant organisms, infection control strategies such as (1) hand hygiene; (2) contact care; (3) following the directions of dedicated hospital personnel infection control policies; (4) environmental sanitation; (5) adherence to decolonization protocols; and (6) establishing monitoring procedures for the diagnosis of asymptomatic patients can be effective as preventive tools (6, 7). Many healthcare facilities propose screening tools for these bacteria. The epidemiological data in every community determine which attendees should be screened. Those admitted directly from long-term acute care institutions and patients admitted from other acute care hospitals should be checked for resistance. Other key indicators to test include hospitalization history (within the previous six months), functional disabilities, and transferring between hospital wards and patients from communities with a high frequency of CRE (8-11).
Although the epidemiological information on CRE in some treatment communities has not been available, it seems necessary to develop the epidemiological model of this type of resistance in each treatment center because this data can be a road map for screening, isolation, and quarantining patients carrying these strains at the beginning of admission to the hospital, which can significantly reduce the transmission of this type of drug resistance between patients and between patients and hospital staff. This strategy seems cost- effective, especially in areas with limited resources. The necessary decisions and measures are taken in each treatment center independently based on the information obtained from epidemiological investigations.
2. Objectives
In this regard, we decided to investigate the prevalence of colonization with Enterobacteriaceae, E. coli, Klebsiella, and Enterobacter resistant to carbapenem and its related risk factors in hospitalized patients in a pediatric referral center in Tehran.
3. Methods
This cross-sectional descriptive study was conducted on 295 stool samples from patients admitted to the hospital for six months in 2021 of which 32% were taken from girls. Patient’s age distribution was 2 - 15 years. All patients hospitalized in different wards, except the neonatal and PICU departments, during the first 48 hours of admission were included in the study. The data of anonymous and unverifiable patients (samples with no name on container) were excluded from the study. After obtaining informed consent, a stool sample was taken from patients in the first 48 hours and sent to the Infection Research Center laboratory with a special patient information form. The sample was cultured in EMB or Mackanki agar medium and incubated for 24 - 48 hours at 37°C. After examining the created colonies, a slide was prepared, and after gram staining, the colonies containing gram-negative bacteria were isolated, and differential tests were performed to identify the bacterial strain, and Enterobacteriaceae strains were isolated. In the next step, an antibiogram was done in Mueller Hinton agar medium using meropenem and imipenem discs by Kirby-Baure method according to CLSI 2021 protocol. Then carbapenemase genes in carbapenem-resistant strains were investigated by using the PCR method for the presence of carbapenemase genes (VIM, IMP, SPM-1, NDM-1, OXA-48). Characteristics of primers that were used are shown in the following Table 1.
| Gene | Base Pairs | Sequence (5’ → 3’) | TM (°C) | PCR Conditions | References |
|---|---|---|---|---|---|
| OXA-48 | 392 | F: CCAAGCATTTTTACCCGCATCKACC | 65.5 | One cycle of initial denaturation at 95°C for 1 min; 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 75°C for 1 min; and one cycle of final extension at 72 for 7 min. | (12) |
| R: GYTTGACCATACGCTGRCTGCG | 61 | ||||
| NDM-1 | 129 | F: CCCCGCCACACCAGTGACANCTC | 75.6 | One cycle of initial denaturation at 95°C for 1 min; 32 cycles of denaturation at 95°C for 30 s, annealing at 61°C for 30 s, extension at 7°C for 1 min; and one cycle of final extension at 72°C for 5 min. | (13) |
| R: GTAGTGCTCAGTGTGGGCAT | 63 | ||||
| VIM | 390 | F: GATGGTGTTTGGTCGCATA | 55.5 | One cycle of initial denaturation at 94°C for 10 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 61°C for 40 s, extension at 72°C for 1 min; and one cycle of final extension at 72°C for 7 min. | (14) |
| R: CGAATGCGCAGCACCAG | 57.2 | ||||
| KPC | 636 | F: CTGTCTTGTCTCTCATGGCC | 60.5 | One cycle of initial denaturation at 94°C for 5 min; 32 cycles of denaturation at 94°C for 35 s, annealing at 62°C for 35 s, extension at 72°C for 32 s; and one cycle of final extension at 72°C for 5 min. | (15) |
| R: CCTCGCTGTGCTTGTCATCC | 62.5 |
In order to collect information, a questionnaire was designed for recording data. The Ethics Committee of Shahid Beheshti University approved this study (Ethical code: IR.SBMU.RICH.REC.1400.019).
3.1. Statistical Analysis
Statistical analysis was performed using SPSS software version 22. The quantitative and qualitative variables were indicated as means and number (percentage), respectively.
4. Results
In this study, 295 stool samples were examined, of which 32% were taken from girls. Patient’s age distribution was 2 - 15 years and 82% of samples had a positive culture with Enterobacteriaceae.
The prevalence of carbapenem resistance among 295 stool samples with a 95% confidence interval (between 31.609-42.629) was reported at 37% with microorganism distribution as: E. coli (59.7%), Klebsiella (23%), Enterobacter (14.6%), Citrobacter (2.7%).
The ratio of resistant to non-resistant microorganisms in patients with underlying disease and a history of hospitalization was 0.48 and 0.48, respectively. Also, the ratio of resistant to non- resistant microorganisms in patients with a history of receiving antibiotics was 0.548 (Table 2).
| Variables | Prevalence (%) | Ratio | Standard Error | 95% Confidence Interval |
|---|---|---|---|---|
| Patients without underlying disease | 8 | 0.366 | 0.062 | 0.254 - 0495 |
| Patients with underlying disease | 29 | 0.486 | 0.037 | 0.413 - 0.559 |
| Patients without a history of hospitalization | 44 | 0.368 | 0.063 | 0.253 - 0.500 |
| Patients with a history of hospitalization | 19 | 0.483 | 0.037 | 0.411 - 0.556 |
| Patients without a history of receiving antibiotics | 14 | 0.356 | 0.044 | 0.274 - 0.448 |
| Patients with a history of receiving antibiotics | 23 | 0.548 | 0.044 | 0.459 - 0.634 |
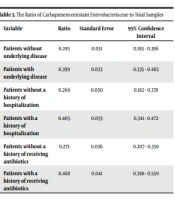
Based on Table 3, the ratio of resistant Enterobacteriaceae to the total samples taken in patients with a history of underlying disease was 0.39. The ratio of resistant Enterobacteriaceae to the total samples taken from patients with a history of receiving antibiotics and patients with a history of hospitalization was 0.468 and 0.405, respectively.
| Variables | Ratio | Standard Error | 95% Confidence Interval |
|---|---|---|---|
| Patients without underlying disease | 0.285 | 0.051 | 0.195 - 0.396 |
| Patients with underlying disease | 0.399 | 0.033 | 0.335 - 0.465 |
| Patients without a history of hospitalization | 0.269 | 0.050 | 0.182 - 0.378 |
| Patients with a history of hospitalization | 0.405 | 0.033 | 0.341 - 0.472 |
| Patients without a history of receiving antibiotics | 0.273 | 0.036 | 0.207 - 0.350 |
| Patients with a history of receiving antibiotics | 0.468 | 0.041 | 0.388 - 0.550 |
According to our findings, the most found gene was OXA-48 (56%), followed by IMP (13%) and VIM (13%). The NDM-1 gene was found in three samples (3%), and the SPM gene was not found in any samples. In 32 samples (29.6%) containing resistant Enterobacteriaceae (in phenotypic test), none of the examined genes were found, which could indicate the presence of other genes or other resistance mechanisms in these strains.
In 13% of resistant samples, more than one resistance gene was found; VIM, IMP (4), VIM, IMP, OXA-48 (1), IMP, OXA-48 (4), OXA-48, VIM, NDM (3), OXA-48, VIM (3), NDM, OXA-48 (1), NDM, VIM (1).
3
5. Discussion
Although the spread of CRE and the resulting infections as a growing concern is a serious threat to public health worldwide, its prevalence and epidemiologic determinants are still unknown in many settings. Infections caused by these bacteria are associated with significant morbidity and mortality and have limited treatment options. Rapid and accurate diagnosis of carbapenem resistance in these spices is important for planning infection control measures (12, 13). Due to the importance of patient-to-patient transmission of resistant strains, especially through contact with colonized stools in high-risk
patients, in the present study, we aimed to investigate the prevalence of community-acquired colonization with Enterobacteriaceae; E. coli, Klebsiella, and carbapenem-resistant Enterobacter to plan the best infection control policies. In this study, the prevalence of carbapenem resistance among stool samples was reported as 37%, and resistance was high in patients with a history of taking antibiotics, a history of frequent hospitalizations, and who had an underlying disease. Among carbapenemase genes, the most found gene was OXA-48, followed by IMP and VIM.
Extensive studies have been conducted in the field of antibiotic resistance patterns in Enterobacteriaceae, which have had different results based on the studied population, sample type, sampling time, and antibiotic resistance assessment method. In the study by Solgi et al., which was conducted to investigate the intestinal carriage of CRE and analyze the risk factors for it in hospitalized adults in Iran, the carriage rate of CRE in hospitalized patients was 37.9% (14). The results of this study were similar to the findings of our study, even though the age group of this study was different, and sampling was done at the beginning of hospitalization to find community-acquired colonization (14). In the study of Peymani and Najafipour, which was conducted on samples taken from hospitalized adult patients in intensive care units in Tehran and Qazvin cities, out of 49 colonies of Enterobacter cloacae isolated by the standard method, 26 (53.1%) of the colonies had a multiple drug resistance pattern based on the Kirby-Baure method, and 1.4% of them showed resistance to carbapenems (15). The lower prevalence of carbapenem resistance compared to the present study can be due to including only one bacterium from the Enterobacteriaceae family in the study, while in the present study, several strains, including E. coli, Klebsiella and Enterobacter, have been investigated in terms of resistance. Moreover, the age group of the study was different. In the study of Shokri et al., a total of 131 strains of E. coli and 43 strains of Enterobacter were isolated from blood and urine cultures of hospitalized patients, of which 79% and 81% were MDR, and 3.3% of E. coli strains and 8.6% of Enterobacter strains were insensitive to carbapenems, which was confirmed with the MIC results (16). The difference in the results of Shokri et al. was due to the type of sample and age group studied. In the present study, stool samples were taken, which are different from blood and urine strains in terms of the type of bacterium and the pattern of resistance (16). In the study conducted by Al Fadhli et al. on rectal swabs from ICU patients during the first 48 hours of admission, out of 590 patients who participated, 58 patients were CRE positive, which showed a prevalence of 9.8% in the screened samples (17). Also, in Rai et al.’s study, among the 242 stool samples taken from patients at the beginning of hospitalization, 9.9% carried carbapenemase-containing strains (18). Although most of these studies were conducted on adult patients, the high prevalence of resistant strains in the samples of children in the first 48 hours in our study can be due to several reasons, such as the effect of incorrect prescription of antibiotics in the treatment of infections, non-adherence to antibiotic-stewardship or transmission of resistance genes by various transmitting agents such as plasmids, bacteriophages, transposons and integrons in our community (19).
The frequency of carbapenemase genes has been different in different studies. The most prevalent gene found in this study was OXA-48. In Pan et al.’s study, which investigated fecal carriage in outpatient children in Shanghai, the blaNDM gene was the main carbapenemase gene found (20). In Mohan et al.’s study that investigated fecal carriage in hospitalized adult patients in India, among 42 CRE isolates, 22 patients carried blaNDM-1, 17 patients had blaVIM gene, and no isolates were positive for blaKPC and blaIMP genes (21). In Solgi et al.’s study, the gene found was OXA-48, followed by blaNDM-1 and blaNDM-7 (14). In this study, similar to our study, the most common gene was OXA-48.
The OXA-48 resistance gene has been reported in more studies compared to other genes. Various studies confirm that many genes encode the carbapenemase enzyme, whose frequency is different in different communities (22, 23). In the present study, more than one resistance gene was reported in 13% of carbapenem-resistant samples, indicating the presence of different plasmids carrying the resistance gene, which can make the treatment more complicated.
In the current study, a high percentage of resistance was observed in patients with antibiotic use, a history of frequent hospitalizations, and patients with underlying diseases, which is in agreement with the results of the study by Yamamoto et al., who reported longer hospitalizations and a history of antibiotic use as risk factors for carrying CRE (24).
In the study of Tran et al., history of hospitalization and history of treatment with carbapenem were also independent risk factors for colonization with CRE (25). In Asai et al.’s study, the results showed that previous hospitalization within 90 days (P = 0.006) and previous antibiotic use within 90 days (P = 0.005) were risk factors for acquiring CRE (26). The results of these studies are also consistent with our study. In the systematic study and meta-analysis by van Loon et al., which was also conducted to investigate the clinical epidemiology of carbapenem-resistant Enterobacteriaceae, it was pointed out that the history of using carbapenem and cephalosporins were the most common risk factors associated with the acquisition of CRE (27). In this study, similar to the current study’s findings, the underlying disease was also a risk factor for resistance (27).
One of the limitations of the present study could be selecting patients from a tertiary university hospital that includes patients with multiple underlying diseases and a frequent history of receiving antibiotics, so the pattern of resistance in this setting may not be representative of the statistical population of children in our community. But considering the purpose of the study, which was to achieve the epidemiological pattern in this hospital, screening patients by this method can decrease colonization rates in our hospital.
5.1. Conclusions
The present study shows a high level of CRE colonization among hospitalized children, indicating the wide distribution of these strains in the community. In general, the high frequency of strains with drug resistance, such as high resistance to carbapenems, indicates the urgent need to review and modify infection control strategies. Considering the high prevalence of carbapenem resistance genes in stool samples colonized with Enterobacteriaceae in our hospital patients, which are located on the plasmids that can be rapidly spread in the hospital environment, it is important for the hospital infection control committee to take preventive measures in order to prevent the spread of these bacteria in our hospital, such as screening stool samples in high-risk patients.