1. Introduction
In 1896, Eppinger isolated an aerobic gram-positive branched filamentous and weakly acid-fast bacillus from a human brain abscess, which was later named Nocardia asteroides. Recently, many new species of Nocardia have been identified with the development of molecular methods, especially gene sequencing. Currently, at least 92 species are known by valid names, of which 54 are important due to clinical conflict reports. Different species of Nocardia have different geographical prevalence, pathogenic characteristics, and antimicrobial susceptibility patterns. Therefore, identifying a specific species of Nocardia is essential for effective treatment, especially in immune-compromised patients (1).
Nocardia is an opportunistic infection with acute, sub-acute, purulent, and diffuse features whose most common site of involvement is the lung. Localized cutaneous or lymphocutaneous infections usually occur after abrasion. Immune-compromised patients such as those with hematologic or solid organ malignancy, autoimmune disorders, HIV infection, diabetes, chronic granulomatous disease, chronic lung disease, and high-dose corticosteroid therapy may have disseminated and systemic Nocardia infection (2).
Periodontitis due to Nocardia infection is not common. A report of purulent infection of the salivary and parotid glands in a 32-year-old woman without immunodeficiency with a history of cleansing her tooth space with a broomstick has been published (3).
In this article, we introduce a child with a history of malignancy and oral abscess caused by Nocardia otitidiscaviarum resistant to common antibiotics.
2. Case Presentation
A 10-year-old child was admitted to the academic and tertiary Pediatric Intensive Care Unit (PICU) with an inability to open his mouth, with an initial diagnosis of dental abscess and the possibility of respiratory problems. He had a history of Pre B cell ALL in the past six years and had relapses and CNS relapses in the past three years. The child had severe secondary immunodeficiency due to a history of recurrent and prolonged neutropenia during relapse and treatment. During his last chemotherapy, the patient was treated with hydrocortisone, methotrexate, vincristine, and oral mercaptopurine. Also, he was receiving anticonvulsant drugs, including levetiracetam and sodium valproate. The child had had swelling in the upper and left jaws one week before hospitalization. The dental abscess was drained by the dentist and then treated with oral antibiotics metronidazole and amoxicillin/clavulanate, but without a proper response. On admission, he was conscious but could not open his mouth, and no mass was visible in the jaw area. The initial signs and heart sounds were normal. The patient had no lymphadenopathy or hepatosplenomegaly. Initial laboratory results are shown in Table 1.
| Lab Test | Result | Reference Range |
|---|---|---|
| Hemoglobin, g/dL | 10.5 | 11.5 - 15.5 |
| White blood cell count, cells/L | 5100 | 4.4 - 11.2 |
| Platelet count, 1000 cells/L | 329000 | 150 - 450 |
| Erythrocyte sedimentation rate, mm/h | 35 | Up to 15 |
| C-reactive protein, mg/L | 41.5 | Up to 5.9 |
| Prothrombin time, s | 12.5 | 10 - 13.6 |
| Partial thromboplastin time, s | 25.8 | 5 - 38 |
| Aspartate aminotransferase, U/L | 13 | 5 - 42 |
| Alanine aminotransferase, U/L | 10 | 15 - 45 |
| Creatinine, mg/dL | 0.5 | 0.5 - 1.2 |
| Calcium, mg/dL | 8.8 | 8 - 13 |
| Lactate dehydrogenase, U/L | 483 | 180 - 1200 |
| D-dimer, ng/mL | 964 | 230 - 480 |
| Fibrinogen, mg/dL | 650 | 150 - 350 |
| Interleukin-6, pg/mL | 15.6 | Up to 9 |
| Ferritin, ng/mL | 1379 | 22 - 322 |
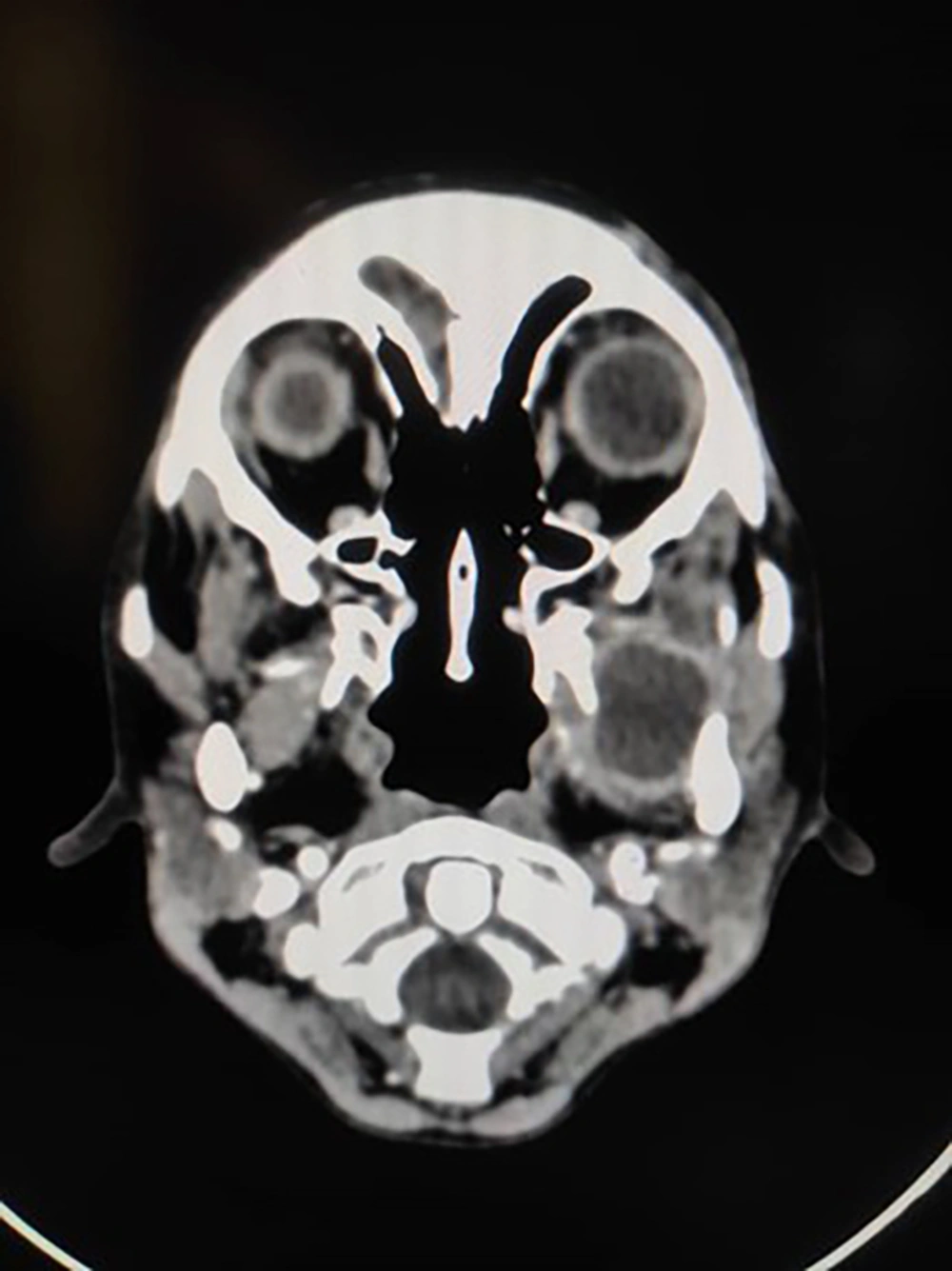
In the CT scan of the mandible and maxilla in the coronal, axial, and sagittal views, occupying space with a cystic appearance and tears in the masticatory cavity were found that invaded the pterygoid muscle. There was no invasion of bone structures. As a result, abscess formation was diagnosed (Figure 1).
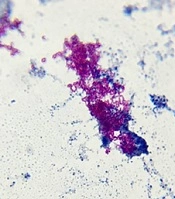
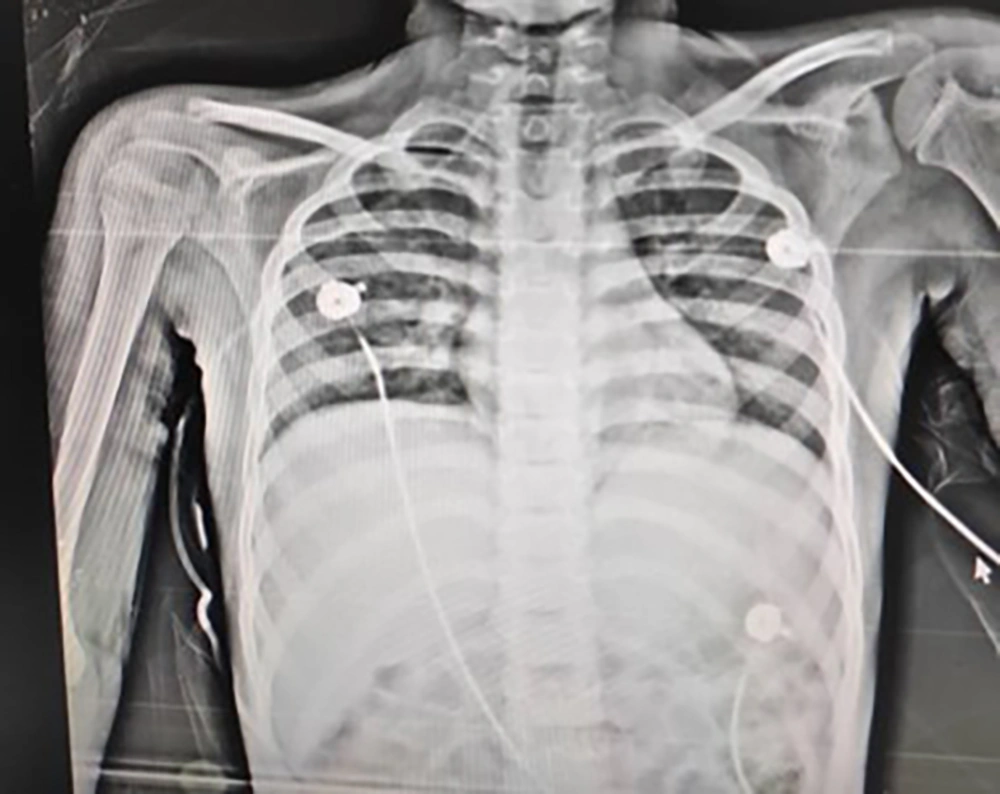
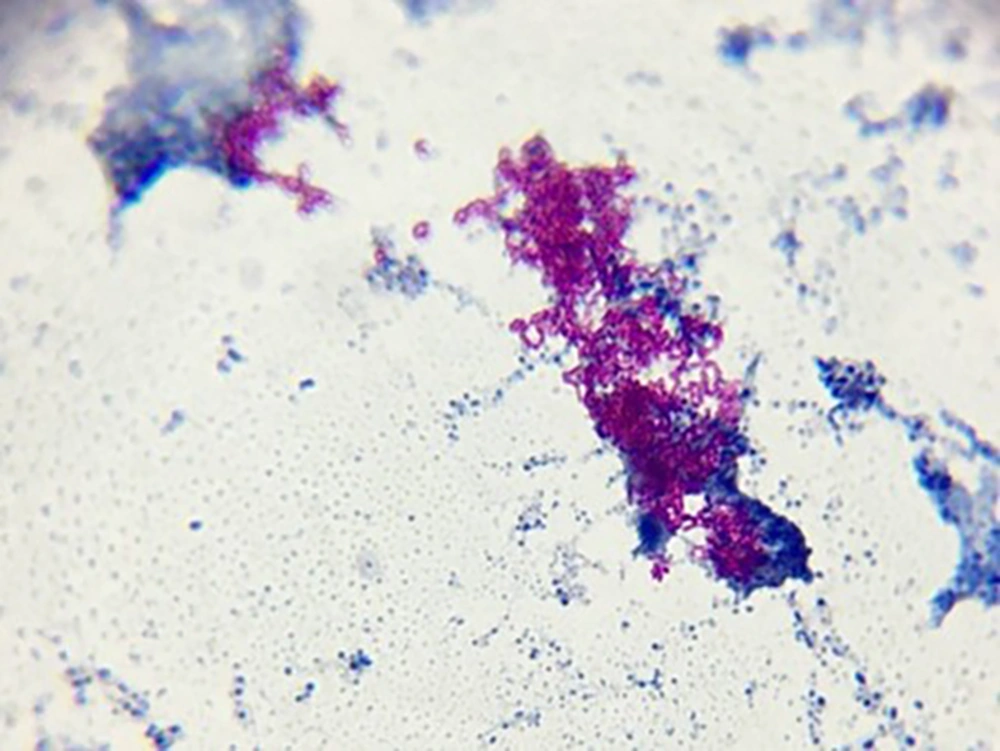
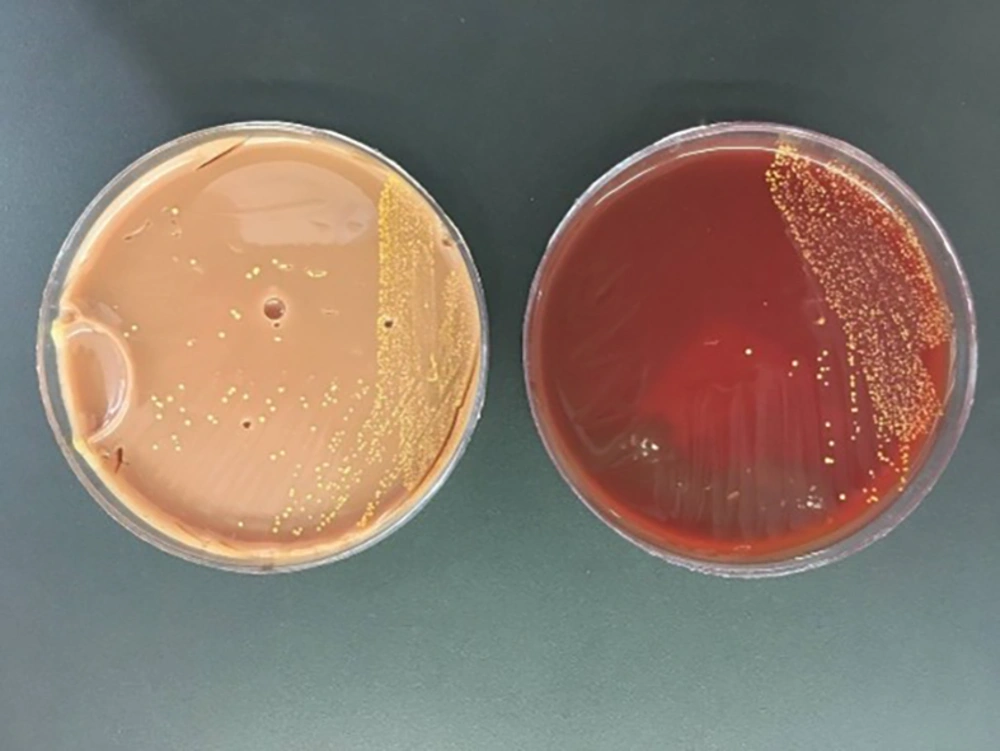
The child was treated with cefepime and clindamycin and became a candidate for abscess drain surgery after ENT counseling. Antibiotics continued after the abscess was drained, but the child developed pulmonary symptoms and needed oxygen. A lung radiograph was performed, and diffuse infiltration was seen, suggesting the possibility of systemic infection (Figure 2). Then, trimethoprim-sulfamethoxazole was added to the treatment. Abscess secretion staining showed gram-positive filaments, and the culture results confirmed Nocardia otitidiscaviarum (Figure 3). Its colonies in the culture medium are seen in Figure 4. The isolated bacteria were identified by Gram and acid-fast staining, catalase, colony and microscopic morphology, and biochemical differential tests. The acid fastness of Nocardia distinguishes it from Actinomycetes. White colonies on culture plates, catalase-positive branching gram-positive bacilli, positive acid-fast staining, and positive partial acid-fast staining indicate Nocardia species. Nitrate reduction, esculin hydrolysis, and antibiogram results determined the species. Based on the phenotypic results, the probable species was Nocardia otitidiscaviarum.
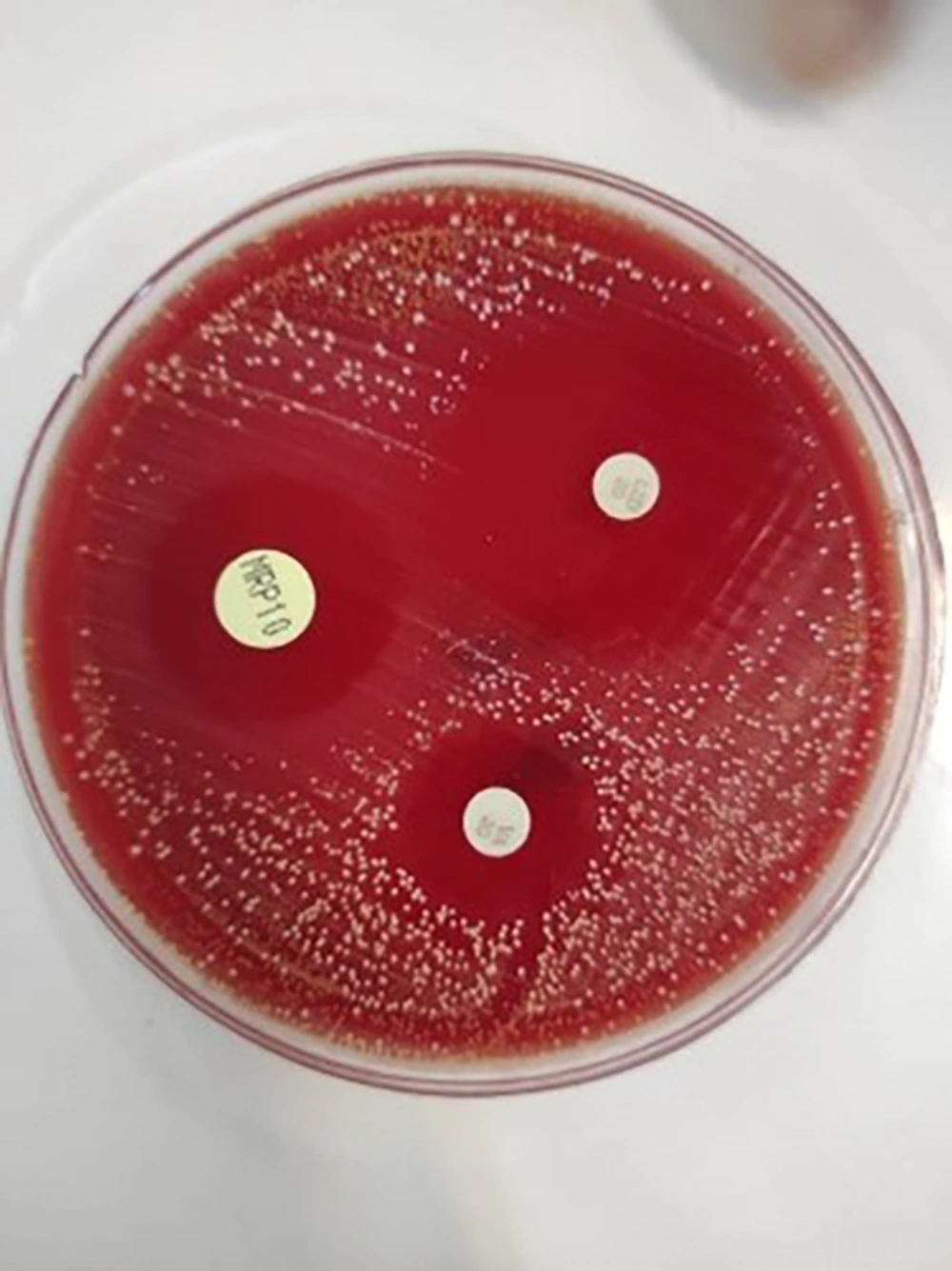
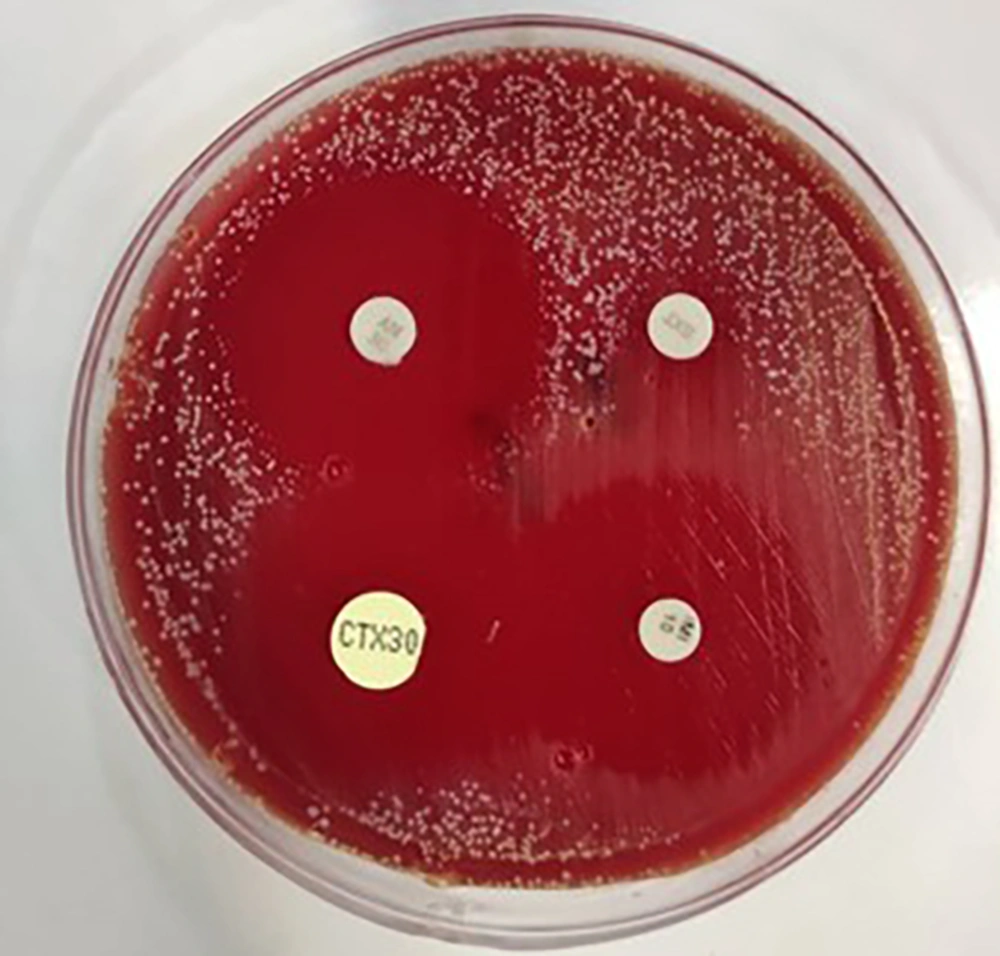
For SMX-TMP resistance screening by disk diffusion method, lawns of each bacterial suspension at a concentration of 0.5 McFarland were made on Mueller Hinton agar using a sterile swab. Then, 23.75/1.25 µg SMX-TMP disks (BD®) were positioned on bacterial lawns and incubated at 37°C for 24 hours (Figure 5). The AST was performed using the disk diffusion method on cation-adjusted Mueller–Hinton agar plates. The results were read after 72 hours of culture. To confirm this SXT TMP-non-susceptible species, the Vitek 2 AST was performed. The inhibition zone diameter was recorded and compared with the final version of the CLSI guideline. The non-susceptible isolate is resistant or intermediate based on an inhibition zone of less than 10 mm (CLSI 2021) (4, 5).
By observing the results of culture and antibiotic susceptibility tests (Figures 5 and 6), it was found that the isolate was sensitive to meropenem, imipenem, linezolid, and amikacin and resistant to cotrimoxazole, ceftriaxone, and tobramycin. As a result, the treatment plan was changed to meropenem, linezolid, and amikacin. The patient recovered after changing the antibiotic but underwent secondary resection due to the accumulation of pus. Parenteral treatment was continued for 4 weeks, and oral linezolid was prescribed for another 4 weeks on an outpatient basis. The inability to open the jaw was followed by a long course of antibiotics and physiotherapy, and the child regained the ability to open her mouth and chew. Cotrimoxazole prophylaxis was started after completing the treatment course and continued due to the patient's immune system deficiency. The child is currently being monitored, and no lesion recurrence has occurred.
This study was approved by the Research Ethics Committee of the School of Medicine, Shahid Beheshti University of Medical Sciences (Approval ID: IR.SBMU.MSP.REC.1401.159).
Informed consent was obtained from the patient's family to report the patient.
5. Discussion
According to a single-center retrospective study and a systematic review of the literature in adults, the most common site of infection with Nocardia is the lungs, with a prevalence of 67%. In extrapulmonary patients, 28% reported evidence of CNS involvement, 17% reported skin or soft tissue disease, and 15% reported pleural disease (6). Lung and brain infections or diffuse infections in immune-compromised patients were most commonly reported in children (7-9). Nocardial face and neck infections are uncommon and are a primary cutaneous lymphatic disease in which a primary lesion is associated with cervical lymphadenopathy and systemic symptoms (10). In an international retrospective study and literature review, primary immunodeficiency was detected in 24.5% of cases with nocardiosis (11). Also, a 15-month-old child with ocular trauma and no history of immunodeficiency has been reported with endophthalmitis due to Nocardia (12).
Our patient has a history of malignancy and recurrent relapses, and chemotherapy and prolonged neutropenia could play an important role in the child developing Nocardia infection. The clinical features and laboratory tests performed on this child were not diagnostic. Contrary to expectations, he did not have cervical lymphadenopathy and damage to the oral mucosa; only imaging and culturing the abscess could help us make the diagnosis. An outpatient dentist treated the child for abscess drainage, but due to the spread of infection, the treatment was unsuccessful and led to an abscess in the masticator area, indicated by swelling, buccal pain, and trismus. In the case of successful drainage, antibiotics are not very helpful, but they should be prescribed for patients with trismus or systemic symptoms or patients with immunodeficiency. Empirical antibiotic therapy for periodontitis involves the co-administration of amoxicillin and metronidazole, but due to the increased antibiotic resistance, especially in immunocompromised children, smear and culture from infected sites is necessary to guide the selection of the appropriate drug (13).
Compared to other Nocardia species, N. otitidiscaviarum is less common and represents 3.1% of cases. This bacterium was resistant to cotrimoxazole in 14.5% of cases (14). Our patient had a recurrence of the abscess but recovered after surgery. He experienced a drop in blood saturation during treatment. Also, lung involvement was detected following a chest radiography. Chest radiographs of such patients show nodules, interstitial infiltration, subpleural plaques, lobar involvement, and lung mass. At the same time, the laboratory diagnosed Nocardia, and the patient's treatment was adjusted accordingly. Due to the noncontiguous involvement of more than two organs, which may or may not include lung involvement, his diagnosis of systemic nocardiosis was confirmed. According to studies, the rate of sensitivity and response to treatment with cotrimoxazole is high, and it is the first treatment choice (15).
The standard empirical treatment for patients with nocardiosis, immunodeficiency, and involvement of more than two organs (excluding the central nervous system) typically consists of trimethoprim-sulfamethoxazole and amikacin or meropenem and amikacin ,However it is important to note that there has been an increase in resistance to trimethoprim-sulfamethoxazole (4). In the case of the child, the antibiogram results indicated resistance to this specific drug. Considering the high sensitivity of Nocardia to carbapenem, amikacin, and linezolid (16), we made the decision to modify the treatment to include amikacin, meropenem, and linezolid. The patient responded well to this adjusted treatment and experienced a successful recovery.
Considering the consequences of the increase of Nocardia species resistant to cotrimoxazole, it is suggested to investigate the effectiveness and safety of long-term prophylaxis with oral linezolid in future research.
5.1. Conclusions
Nocardia should be considered a causative agent of pyogenic infections in immunocompromised patients who do not respond well to empiric therapy. Due to increased strains resistant to common antibiotics, treatment should be based on antibiogram results. The duration of treatment in these patients is long, and the prevention plan for opportunistic infections in immunocompromised patients is very important.