1. Background
Almost four years have passed since the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) a pandemic (1). Throughout this period, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for COVID-19, has led to a wide array of manifestations and complications, including pulmonary involvement, acute respiratory distress syndrome (ARDS), multi-organ failure, and death (2).
One manifestation of SARS-CoV-2 infection is the multisystem inflammatory syndrome in children (MIS-C) (3). This syndrome, observed in children and adolescents, is temporally associated with COVID-19 and is characterized by laboratory evidence of inflammation, multisystem organ involvement, fever, and epidemiological or laboratory evidence of SARS-CoV-2 infection in individuals under 19 years of age (4, 5). Its etiology remains uncertain, though it is proposed to be a post-infectious manifestation stemming from an abnormal immune response (6). Furthermore, SARS-CoV-2 can lead to a broad spectrum of neurological involvements, including Guillain-Barre syndrome, transverse myelitis, reversible cerebral vasoconstriction syndrome, and encephalitis (7, 8).
Additionally, while studies have identified various viruses like Epstein–Barr virus (EBV) and Varicella zoster virus (VZV) in COVID-19 patients, there has not been a significant association found between COVID-19-associated viruses and neurological complications (9, 10).
As the number of MIS-C cases grows, the occurrence of life-threatening neurological complications, such as encephalitis, has increased (6).
2. Objectives
Given the link between viral reactivation and SARS-CoV-2 infection, along with the presentation of encephalitis as a manifestation of SARS-CoV-2 infection, our study aims to investigate the reactivation of different viruses in children experiencing concurrent COVID-19 and encephalitis.
3. Methods
3.1. Patients and Sample Collection
This study is an analytical cross-sectional research project conducted at Imam Hussain Pediatric Hospital in Isfahan, Iran, from February 2020 to May 2021. It included patients diagnosed with COVID-19-associated MIS-C exhibiting central nervous system (CNS) involvement as part of the MIS-C criteria organ impacts.
The diagnosis of MIS-C was established by fulfilling all the following criteria (11), in addition to the prior identification of SARS-CoV-2 through a nasopharyngeal polymerase chain reaction (PCR) test (PT.COVID.100, PISHTAZ TEB, 2020) or an elevated antibody titer related to COVID-19: (a) Fever (lasting more than 24 hours or documented to be ≥ 38 Celsius); (b) laboratory evidence of inflammation, including reduced lymphocyte and albumin levels or elevated counts of neutrophils, CRP, ESR, ferritin, and LDH; (c) illness necessitating hospitalization; (d) involvement of multiple systems (≥ 2 organs), such as cardiac, renal, respiratory, gastrointestinal, circulatory, skin, and nervous systems; (e) absence of any alternative diagnoses that could explain the symptoms.
Central nervous system involvement was defined by one major criterion and at least two minor criteria (12): The major criterion includes altered mental status (characterized by a reduced or altered level of consciousness, lethargy, or personality changes) lasting ≥ 24 hours without an identifiable alternative cause; the minor criteria include fever ≥38° C (100.4°F) recorded within 72 hours before or after presentation; generalized or partial seizures not fully attributable to a pre-existing seizure disorder; new-onset focal neurologic findings; CSF WBC count ≥ 5/mm^3; brain parenchyma abnormalities on neuroimaging indicative of encephalitis that are either new compared to previous studies or appear acutely onset; and abnormalities on electroencephalography consistent with encephalitis, not explained by another condition.
Cerebrospinal fluid (CSF) samples were collected to exclude other potential diagnoses, such as bacterial infections. Cerebrospinal fluid cultures and smears were negative for all patients. All patients were treated according to the Iranian treatment protocol for children with MIS-C (13), which included 2 g/kg of intravenous immunoglobulin (IVIG) administered over two consecutive days, 5 mg/kg of aspirin taken orally daily for 8 weeks, 1 mg/kg of enoxaparin administered subcutaneously daily during hospitalization, 10 mg of famotidine taken orally daily, and 2 mg/kg of prednisolone given daily during the hospital stay. The prednisolone dose was tapered over 4 weeks following discharge. The samples were collected with stringent sterile techniques to prevent contamination. PCR tests for viral particles were performed retrospectively on the patients' samples using two different methods, both confirming the presence of viral particles.
Written informed consent was obtained from the patients' parents after the study was fully explained to them. This study received approval from the ethics committee of Isfahan University of Medical Sciences, with the ethical code number: IR.MUI.MED.REC.
3.2. Data Collection
Initial CSF analysis involved measuring white blood cells, red blood cells, glucose, and protein levels. The collected CSF samples underwent PCR testing to identify viral genomes, including those of the varicella-zoster virus (VZV), cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein-Barr virus (EBV), adenovirus, and influenza virus. Demographic, clinical, laboratory, and follow-up data were collected for all cases. Data collection followed the guidelines outlined in the WHO record paper for MIS-C, titled Global COVID-19 Clinical Platform (14).
Data were gathered from medical records following patient hospitalization. Senior pediatric residents verified all data. Furthermore, a trained radiologist, who was blind to the patients' conditions and severity, reported on brain magnetic resonance imaging (MRI) results. Follow-up visits at four-week and six-month intervals were conducted to assess the patients' conditions and any complications. The Pediatric Quality of Life Inventory (PedsQL) was utilized to evaluate quality of life during the six-month follow-up. The Persian version of the PedsQL is consistent with the original version and varies according to the child's age (15, 16).
4. Results
Thirteen patients diagnosed with MIS-C and CNS involvement, presenting as encephalitis, were admitted to our hospital from February 2020 to May 2021. Prior to receiving antibiotics, intravenous immune globulin (IVIG), or corticosteroids, all patients underwent a lumbar puncture. The viral genome was detected via PCR in the CSF of six patients. The median age was 44 months, with eight patients being male. None had any pre-existing neurological or systemic diseases, and all were fully vaccinated according to the Iranian vaccination protocol. No cases of immunodeficiency were observed. Among the patients with positive CSF PCR for viruses (PCR+ group), four were male (compared to 3 out of 7 in the PCR- group, P-value = 0.592). The median age of the PCR- group was 46 months, with four being male. A positive SARS-CoV-2 CSF PCR case was found among all cases.
The most common initial symptoms in both the PCR+ and PCR- groups were nausea (6 and 7 cases, 100%), seizures (5 and 5 cases, 83% and 71% respectively), diarrhea, and loss of consciousness (3 and 4 cases, 50% and 57% respectively). Physical examinations revealed tachycardia in 2 cases in the PCR+ group and 4 in the PCR- group. Tachypnea was reported in only one patient from the PCR+ group. Skin rash was observed in 5 cases in the PCR+ group and 4 in the PCR- group (83% and 57%, respectively). Neck rigidity and Brudzinski's sign were noted in 2 and 3 patients of the PCR+ and PCR- groups, respectively. Lymphadenopathy and edema were present in two patients from each group. Laboratory data comparison between the PCR+ and PCR- groups is detailed in Table 1.
| Parameter | Group | P-Value b | |
|---|---|---|---|
| PCR+ (of 6 Cases) | PCR- (of 7 Cases) | ||
| Lymphopenia | 4 (66.6) | 5 (71.42) | > 0.999 |
| Leukopenia | 0 | 0 | > 0.999 |
| Elevated C-reactive protein | 6 (100) | 6 (85.71) | > 0.999 |
| Elevated ferritin | 2 (33.3) | 3 (42.85) | > 0.999 |
| Elevated d-dimer | 3 (50) | 3 (42.85) | > 0.999 |
| Anemia | 3 (50) | 2 (28.57) | 0.592 |
| Thrombocytopenia | 4 (66.6) | 3 (42.85) | 0.592 |
| Thrombocytosis | 2 (33.3) | 1 (14.28) | 0.559 |
| Positive blood cultures | 0 | 0 | > 0.999 |
| Abnormal liver function tests | 2 (33.3) | 4 (57.14) | 0.592 |
| Elevated troponin | 3 (50) | 4 (57.14) | > 0.999 |
| Elevated lactate dehydrogenase | 2 (33.3) | 3 (42.85) | > 0.999 |
| CSF analysis | |||
| Low glucose (< ½ simultaneous blood glucose) | 4 (16.6) | 3 (342.85) | 0.592 |
| Protein (> 40 mg/dl) | 0 | 0.461 | |
| Leukocyte (≥ 5 cells/mm3) | 4 (66.6) | 2 (28.57) | 0.286 |
| Neutrophil dominancy | 2 (33.3) | 0 | 0.192 |
a Values are expressed as No. (%).
b Fisher’s exact test.
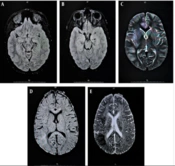
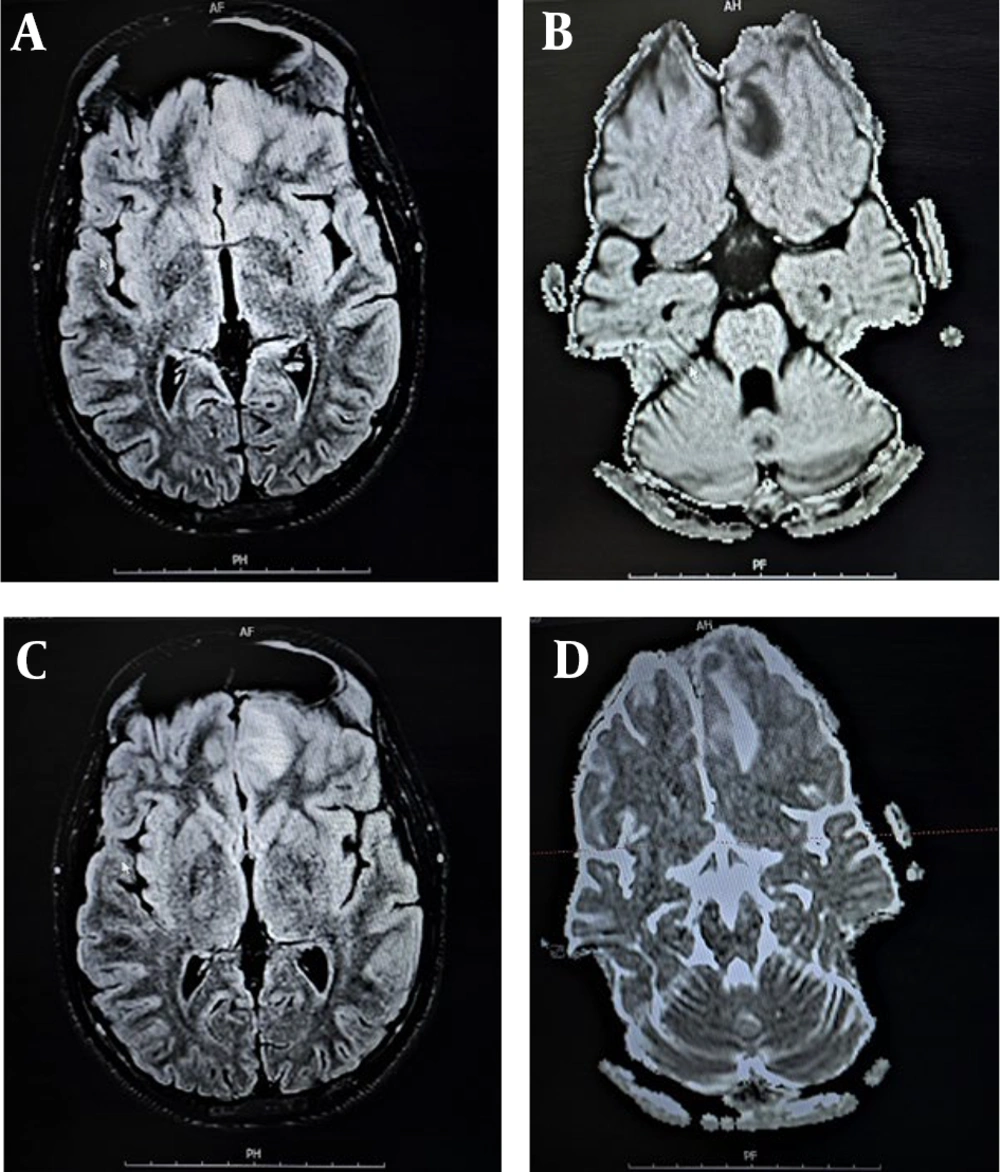
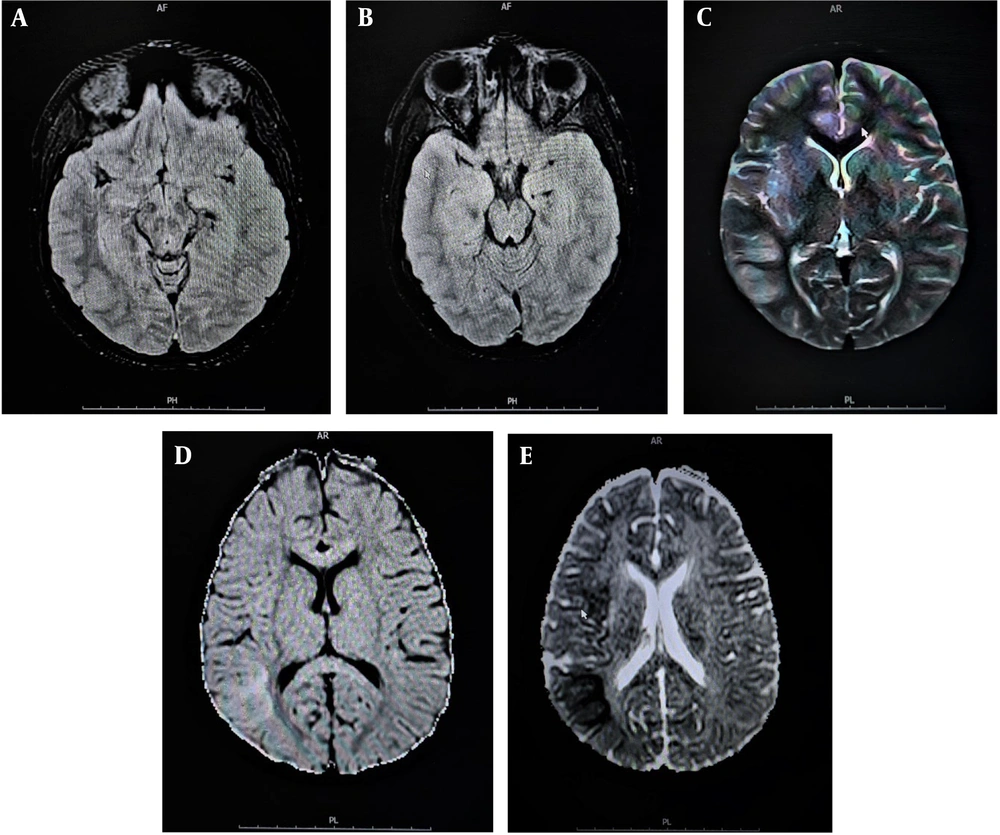
Cerebrospinal fluid smears and cultures for all patients were negative. However, the viral genome was detected via PCR in the PCR+ group, with EBV found in four cases and HSV in two cases. Brain imaging was conducted for all patients, with Figures 1 and 2 displaying MRI scans for patients 1 and 4, respectively. Additionally, electroencephalography, performed for three patients in the PCR+ group, showed normal results. Table 2 presents demographic, clinical, laboratory, and imaging findings for the PCR+ group.
| Patient ID | Age | Sex | Symptoms | Signs | Lab Tests | CSF Analysis | CSF PCR | Brain MRI |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 y | Male | Fever, Nausea, Seizure, Diarrhea Headache, Loss of consciousness | Tachycardia, Lymphadenopathy | Elevated CRP, Lymphopenia, Anemia, Thrombocytopenia | WBC: 180 /mm3; PMN: 20%; High Protein; Low Glucose | EBV | Left frontal leptomeningitis and cerebritis |
| 2 | 1 y 5 mo | Female | Fever, Nausea, Seizure, Headache | Hand and foot edema, Skin rash, Conjunctivitis | Elevated CRP, Thrombocytosis, Elevated ferritin and D-dimer | Normal CSF | HSV | Normal |
| 3 | 3 y 6 mo | Male | Fever, Nausea, Diarrhea | Hypotension, Skin rash, Neck rigidity, Conjunctivitis, Agitation | Elevated CRP, Anemia, Thrombocytosis, Elevated troponin and LDH | WBC: 5 /mm3; PMN: 60%; Low Glucose | EBV | Normal |
| 4 | 1 y 11 mo | Male | Fever, Nausea, Seizure, Loss of consciousness | Tachycardia, Tachypnea, Seizure, Skin rash | Elevated CRP, Lymphopenia, Anemia, Thrombocytopenia; Elevated troponin, LDH, AST, ALT and D-dimer | WBC: 11 /mm3; PMN: 90%; Low Glucose | EBV; SARS-CoV-2 | Encephalitis |
| 5 | 3 y 10 mo | Female | Fever, Nausea Diarrhea, Seizure | Skin rash, Conjunctivitis, Lymphadenopathy | Elevated CRP, Lymphopenia, Thrombocytopenia | Normal CSF | EBV | Normal |
| 6 | 12 y | Male | Fever, Nausea, Seizure, Loss of consciousness | Neck rigidity, Skin rash, Conjunctivitis, Lymphadenopathy, Hand and foot edema, Brudzinski’s sign, Hypotension | Elevated CRP, Lymphopenia, Thrombocytopenia, Elevated troponin, D-dimer, ferritin, LDH, ALT and AST | WBC: 50 /mm3; PMN: 40%; Low glucose | HSV | Normal |
Abbreviations: Y, years, mo, months; CRP, C- reactive protein; WBC, white blood cell; CSF, cerebrospinal fluid; PMN, polymorphonuclear; EBV, Epstein-Barr virus; HSV, herpes simplex virus; CT scan, computed tomography scan; MRI, magnetic resonance imaging; LDH, lactate dehydrogenase; AST, Aspartate transaminase; ALT, Alanine transaminase; CRP, C-reactive protein.
Given that the detection of viral genomes in CSF samples was done retrospectively, all patients were treated according to the Iranian treatment protocol for children with COVID-19, irrespective of the presence of concurrent CSF infections; no patient received antiviral therapy. The median [first and third quartiles] duration of hospitalization was 8.5 days (range 6 - 17 days). The illness severity in the cases studied was moderate, necessitating hospitalization, but none required ICU admission or experienced shock.
All patients received IVIG at a dose of 2 mg/kg. At the 4-week follow-up, case 4 reported experiencing seizures. Additionally, speech disorders and hearing loss were noted during the 4-week follow-up for case 6. These two complications had resolved by the 6-month follow-up. No other complications were reported at either the four-week or six-month follow-ups. Quality of life assessments using the PedsQL indicated that these patients' quality of life was normal after six months.
5. Discussion
Recent studies have indicated that neurological involvement might be common in patients with MIS-C, although the clinical syndrome remains poorly defined. Some patients with MIS-C can exhibit acute encephalitis characterized by rapid-onset encephalopathy, occasionally accompanied by focal neurological signs (6, 17). In our study, we assessed 13 MIS-C patients who exhibited CNS involvement manifesting as encephalitis, an organ involvement criterion of MIS-C. Cerebrospinal fluid (samples from these patients were subjected to PCR testing for various viruses, revealing the presence of 2 types of viruses in 6 patients' CSF samples: EBV in four cases and HSV in two cases. There have been numerous reports of latent virus reactivation during infections or other diseases (9, 18, 19). EBV reactivation has been associated with autoimmune diseases or cancers (9) and has also been reported in COVID-19 patients (18). The prevalence of EBV DNAemia in healthy individuals varies across studies (20, 21). Brooks et al. reported EBV DNAemia in 20% of patients with COVID-19 compared to 14% in the healthy population (22). Bernal and Whitehurst found that EBV DNA prevalence was higher among COVID-19 positive patients (27.1%) compared to healthy individuals (12.5%) (23). There have been suggestions that EBV reactivation may relate to post-COVID-19 syndrome or a severe course of COVID-19 (18, 24). Additionally, it has been shown that the transfection of COVID-19 anti-spike protein is associated with the reactivation of gamma-herpesviruses (25, 26). However, a recent study by Hoeggerl et al. reported no difference in EBV-specific antibody levels between patients with post-COVID-19 syndrome and healthy individuals (27).
Additionally, case studies have indicated that herpes simplex virus (HSV) reactivation is not exclusive to COVID-19 patients but also occurs in those with other viral and bacterial infections. Luyt et al. observed that HSV reactivation rates in patients with COVID-19 exceed those in patients with influenza (28). Chiesa et al. noted mucocutaneous herpes simplex virus reactivation in adult COVID-19 patients (29). Shanshal and Ahmed reported that individuals with COVID-19 might experience up to three HSV outbreaks during their illness (30). Alharthy et al. documented a case of HSV and SARS-CoV-2 co-infection in an HIV-positive patient (31), and Patel et al. described a case of encephalitis with varicella-zoster virus (VZV) in a COVID-19 patient (10). Previously, HSV reactivation was also seen during Mycoplasma infections (32).
It's important to recognize that MIS-C is linked to immune dysregulation, including lymphopenia and elevated levels of interleukin-1 and TNF-alpha (33). Such immune dysregulations and inflammatory cytokines may trigger the reactivation of herpes simplex virus from latency to epithelial surfaces. Consequently, HSV replication and its lytic stage on epithelial surfaces lead to the presence of HSV DNA in the blood and CSF. Consistent with previous findings, we report pediatric cases with MIS-C alongside EBV or HSV reactivation in the CSF, treated following the MIS-C protocol. An appropriate therapeutic regimen for MIS-C was administered to our patients, but antiviral treatment for encephalitis was not pursued. Considering the favorable 6-month outcomes and the absence of virus-specific manifestations, such as typical skin lesions, it appears that detecting viral genomes results from virus reactivation rather than primary infection.
Our study faced several limitations, including missing data due to its retrospective design, suggesting the need for future prospective studies. Additionally, the relatively small sample size could be overcome by conducting multi-center studies. Given the novelty of MIS-C and its manifestations, extended follow-up for these patients is warranted. Moreover, additional diagnostic evaluations may be necessary.
5.1. Conclusions
This study identified reactivation of viruses, notably EBV and HSV, in pediatric patients with concurrent multisystem inflammatory syndrome and encephalitis associated with SARS-CoV-2 infection. These findings suggest virus reactivation rather than primary infection. Thus, virus reactivation in patients with MIS-C could open new avenues for future research and the development of therapeutic strategies.