1. Background
Coronavirus disease 2019 (COVID-19), resulting from severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), can lead to acute respiratory distress syndrome (1, 2).
Patients suffering from COVID-19 have been subjected to viral, bacterial, and fungal co-infections, making their condition much more difficult to diagnose, treat, and prognosis of COVID-19 (3). They have an elevated risk of contracting fungal co-infections because of hospitalization, underlying conditions, and medicinal regimens, including steroids and antibiotics (4). In fact, co-infections caused by fungi have been observed in 3.7% of COVID-19 patients. Moreover, the rate is considerably higher for those admitted to the intensive care unit (ICU) (9.6%). Aspergillus and Mucor species (spp.) are among the most frequently reported causes of COVID-19-associated fungal infections (CAFIs) (3).
Aspergillus spp., as an opportunistic fungal agent, can lead to invasive pulmonary aspergillosis (IPA), a serious condition. Invasive pulmonary aspergillosis is noted in COVID-19 patients with clinical manifestations similar to IPA in influenza patients and is termed COVID-19-associated pulmonary aspergillosis (CAPA) (5).
Mortality rates of as high as 54% are attributed to mucormycosis. This high mortality rate is caused mainly by late diagnosis and treatment of the disease (6).
Multiple factors can contribute to the pathogenesis of COVID-19-associated mucormycotic (CAM) (7). Extremely ill patients who are on mechanical ventilation and oxygen therapy or those staying for long periods in ICUs are at higher risks of mucormycosis. In addition, high mortality rates are reported among patients with intracranial extension (8). Other risk factors for mucormycosis in COVID-19 patients include corticosteroids and broad-spectrum antibiotics (3). Since the beginning of the pandemic in Iran, multiple CAFI cases from all over Iran have been reported (3).
2. Objectives
Due to diagnostic limitations, it is difficult to recognize between invasive fungal infections and fungal identification during the COVID-19 pandemic. On the other hand, some studies have informed the incidence of invasive candidiasis in patients with COVID-19 (9, 10). As there has been no study on fungal identification from mini-BAL in children with COVID-19, this study aimed to determine the frequency of fungal identification in ICU-hospitalized children from April 2021 to February 2022.
3. Methods
3.1. Study Design
A cross-sectional descriptive study was performed on mini-BAL samples from children confirmed positive for COVID-19 admitted to ICU with a ventilator at Mofid Children's Hospital, Tehran, Iran, from April 2021 to February 2022. Children's information was recorded after obtaining parental consent to enter the study. The demographic characteristics, such as age, gender, symptoms (fever, shortness of breath, cough, and decreased level of consciousness), previous history of COVID-19, and underlying disease were recorded for all children by questionnaire forms.
Criteria for entering the study were all children with COVID-19 (either with a positive molecular test or lung CT involved) who were admitted to the COVID-ICU of Mofid Children's Hospital in one year, but cases where the file information was incomplete were excluded from the study.
3.2. Confirmed COVID-19 Cases
COVID-19-positive status was confirmed using two methods of real-time PCR after RNA extraction on mini-BAL of suspected children and lung involvement on chest CT scan that confirmed pneumonia with COVID-19 signs. Mini bronchoalveolar lavage (mini-BAL) is a blind, non-bronchoscopic procedure used to obtain samples from the lower respiratory tract of patients on mechanical ventilation. The mini-BAL collection was performed by a sterile long suction catheter of size 12 French inserted through the ET and blindly advanced into the distal airways till resistance was felt, then the catheter was wedged in that position. Twenty milliliter of sodium chloride 0.9% was instilled through the catheter, and aspirate was collected in a sterile polypropylene collector tube container by suction. After these proceedings, the probe was delicately removed using turning movements.
3.3. Molecular Diagnosis of Fungi
Fungal DNA was extracted from the mini-BAL with a DNA extraction kit (Sambio; South Korea). Nested PCR was made with two sets of primers for Mucor app. and Aspergillus fumigatus to detect 175-bp and 236-bp DNA fragments, respectively (8, 9). The outer and inner primers of Mucor app. and A. fumigatus are listed in Table 1. PCR was performed with a thermocycler (5530 Mastercylcler, Eppendorf, Germany) under the following thermal conditions: a denaturation step for 5 min at 94°C, followed by 35 cycles of denaturation for 30 sec at 94°C, annealing for 30 sec at 55°C for Mucor and 63°C for A. fumigatus, extension for 1 min at 72°C, a final extension step for 8 min at 72°C (11).
| Genus and Primer | Sequences |
|---|---|
| Mucor spp. | |
| Outer | |
| ZM1 | 5´-ATT ACC ATG AGC AAA TCA GA-3´ |
| ZM2 | 5´-TCC GTC AAT TCC TTT AAG TTT C-3' |
| Inner | |
| ZM1 | 5´-ATT ACC ATG AGC AAA TCA GA-3´ |
| ZM3 | 5´-CAA TCC AAG AAT TTC ACC TCT AG-3' |
| Aspergillus fumigatus | |
| Outer | |
| AFU5S | 5´-AGG GCC AGC GAG TAC ATC ACC TTG-3´ |
| AFU5AS | 5´-GG G(AG)GT CGT TGC CAA C(CT)C (CT)CC TGA-3´ |
| Inner | |
| AFU7S | 5'- CGG CCC TTA AAT AGC CCG-3' |
| AFU7AS | 5´-GA CCG GGT TTG ACC AAC TTT-3´ |
Primers Used in Nested PCR
3.4. Statistical Analysis
All statistical analyses were performed using SPSS software (ver. 15.0, USA). An independent t-test was utilized to compare positive BAL PCR for Mucor spp. and Mucor negative patients regarding chest CT scan score and time spent under a ventilator. The data's normality was checked using the Shapiro-Wilk test, and considering that the data had a normal distribution, parametric tests such as the independent t-test were used.
3.5. Ethical Considerations
The ethics committee of the Research Institute for Children's Health IR.SBMU.RICH.REC.1400.042 at Shahid Beheshti University of Medical Sciences approved this study.
4. Results
The majority of children with COVID-19 positive status (n = 58/100; 58%) were male (a male: female ratio of 1.38) in the study sample, and the mean age of the patients was 48.3 months (age range: 4 - 120 months). Among all COVID-19 patients (n = 100), the highest prevalence of fungal identification was seen among those with decreased consciousness and heart diseases (Table 2). Moreover, 12 patients with fungal identification received similar medications, as demonstrated in Table 2.
| Characteristic | Male; No. (%) | Female; No. (%) |
|---|---|---|
| Age range (mo) | ||
| < 40 | 7 (70) | 1 (50) |
| 41 - 80 | 1(10) | - |
| 81 - 120 | 2 (20) | 1 (50) |
| Total | 10 (83) | 2 (17) |
| Symptoms | ||
| Decreased consciousness | 3 (25) | |
| Cough | 3 (25) | |
| Shortness of breath | 2 (16.6) | |
| Fever | 1 (8.3) | |
| Underlying conditions | ||
| Heart diseases | 5 (41.7) | |
| Underlying malignancy | 4 (33.4) | |
| Digestive diseases | 3 (25) | |
| Neurological diseases | 1 (8.3) | |
| Administered medications | ||
| Corticosteroids | 12 (100) | |
| Antibiotics | 12 (100) | |
| Antivirals | 11 (92) | |
Distribution of Patients with Mucor Identification Based on Different Characteristics
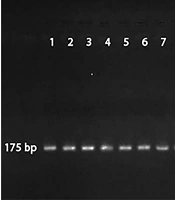
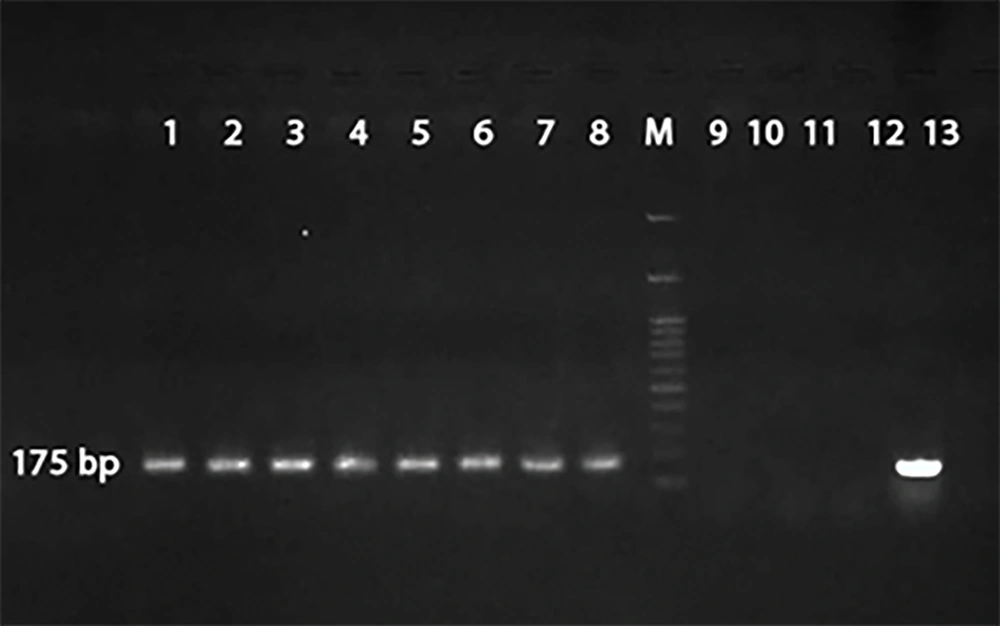
Based on the nested PCR and production of DNA fragments, out of 100 mini-BAL samples, 12 samples (male: 10 and female: 2) were positive BAL PCR for Mucor spp. (175-bp). In this survey, A. fumigatus (236-bp) was not found (Figure 1).
Positive BAL PCR for Mucor spp. in 11% (n: 8) of patients with lung involvement (confirmed by CT scan) and 14% (n: 11) of antiviral recipients, respectively. As demonstrated in Table 3, positive BAL PCR for Mucor spp. patients had significantly higher chest CT scan scores and spent more than double as much time under a ventilator compared to Mucor-negative patients.
| Variables and Group | Positive BAL PCR for Mucor spp. (Mean ± SD) | Mucor Negative (Mean ± SD) | Independent t-Test | ||
|---|---|---|---|---|---|
| T | df | P | |||
| CT. Score | 9.83 ± 0.93 | 5.30 ± 2.90 | 5.32 | 78 | < 0.001 |
| Ventilator duration | 12.75 ± 3.81 | 6.16 ± 2.14 | 8.59 | 78 | < 0.001 |
CT Scan Scores and Time Spent Under a Ventilator for Positive BAL PCR for Mucor spp. and Mucor Negative Patient, Independent t-Test Was Used
5. Discussion
Fungal infections are reported as important co-infections among patients with Coronavirus disease. The most frequent fungal infections reported among COVID-19 patients are invasive aspergillosis, mucormycosis, and candidemia (8). Studies conducted on secondary fungal infections among COVID-19 patients are mostly aimed at older age groups (3, 7, 8, 12-15). However, recognizing invasive fungal infection or just fungal identification is not simple.
Our results showed fungal identification in 11% (n: 8) of the patients with lung involvement and 14% (n: 11) of those who had received antiviral treatment. And those with heart diseases (41.7%; n: 5) and underlying malignancies (33.4%; n: 4) were most likely to isolate fungi. Moreover, higher CT scan scores and time under ventilation were recorded for positive BAL PCR for Mucor spp. patients.
Mucor spp., which belongs to the order Mucorales, can cause invasive fungal infections (16). Although a study conducted before the COVID-19 pandemic showed an almost 2.5-fold increase in the incidence of mucormycosis in Iran from 2008 to 2014, the prevalence of this fungal infection has grown 50 times globally after the COVID-19 pandemic (3).
Because of the highly transmissible nature of SARS-CoV-2 during the pandemic, bronchoscopies and BAL sampling are not usually performed at medical centers (1). The current study, however, used mini-BAL samples for fungal identification. Of 100 mini-BAL samples from COVID-19 patients, 12% of cases (n: 12) turned positive BAL PCR for Mucor spp. Among the positive cases, there were 83% males (n: 10) and 17% females (n: 2), very much in line with other studies, where mucor presence is more common among males (3, 7, 8, 12, 15, 17, 18). In this study, due to primers specific to A. fumigatus, other Aspergillus species were not detected, which is one of the study's limitations.
Aspergillosis, another frequent fungal co-infection of Coronavirus disease, has been reported in 7.7% - 27.7% of critically ill COVID-19 patients. COVID-19-associated pulmonary aspergillosis inflicts as high as 35% of COVID-19 patients in the ICU, causing a mortality rate of 54.9% (3). Our study, however, reports no cases of A. fumigatus identification among hospitalized children with COVID-19. Similar results were reported in a medical center in the midwestern USA, where CAPA was uncommon and affected only 1% of patients (14). Likewise, a study conducted in Switzerland reported just 2.5% of cases of CAPA in severely ill COVID-19 patients (19). This data contrasts with several European studies, which have reported a 19% - 33% prevalence of CAPA among critically ill COVID-19 patients (14). The CAPA incidence differs among studies and probably reflects the diagnostic guidelines and the population under study (20). These various frequencies of CAPA in different studies could be due to different studied populations and the state of hygiene in the studied medical centers.
Despite the recognized prevalence of IPA in the ICU, the diagnosis is usually complicated due to the possibility of colonization (12). The differentiation between the angio-invasive disease and colonization in CAPA can be challenging since serum biomarkers are usually negative, indicating colonization (13). Based on clinical findings, the incidence of CAPA varies from 10% to 28%. In comparison with clinical diagnosis, autopsy suggests a lower prevalence of invasive disease, which can hint that some of the cases diagnosed clinically are, in fact, cases of colonization (20).
Similar to the results of the current study, major predisposing factors for fungal identification among COVID-19 patients include the excessive use of mechanical ventilation (P < 0.001) as part of their COVID-19 treatment (1-8, 14, 17, 20). Similarly, in this study, all cases of fungal identification among COVID-19 patients were on a corticosteroid regimen and spent a markedly longer time (P = 0.001) under mechanical ventilation. These conditions are risk factors for fungal identification in COVID-19 patients.
Finally, the significant increase in chest CT scores among COVID-19 patients with mucormycosis, compared to Mucor-negative patients, suggests that Mucor spp. Identification of COVID-19 might have a synergic effect on pulmonary symptoms. Also, we face two separate situations in the current study: 1, Severity of COVID-19 and more effect on the lungs because of the identification of Mucor spp.; and 2, the identification of Mucor spp. effect on the patient's lung, and we observed an increase in the CT score of these patients. Both of these possibilities can be investigated, and it is suggested to separate these two conditions from each other in future studies. There is still dispute about the role of fungal infections in the progress of pneumonia.
A significant limitation of the study is that due to COVID-19 infection control to limit the spread of infection, we could not re-sample the positive COVID-19 children, so it was impossible to culture the samples for a more detailed examination of the fungal species. Another limitation of our study is that there is no recognition between fungal invasives and fungi colonization. Other limitations of this study were missing testing galactomannan in blood and BAL, missing testing concurrent aspergillosis blood PCR, and missing BAL culture at the first sampling time.
5.1. Conclusions
To the best of our knowledge, this is the first study on children with COVID-19 to examine the identification of fungi. Patients with mechanical ventilation, prolonged ICU stays, cardiovascular diseases, and malignancies may be more susceptible to fungal identification. So, clinicians should consider the importance of the differential diagnosis of fungal co-infections, especially among patients with high-risk factors.