1. Background
Staphylococcal scalded skin syndrome (4S) is caused by Staphylococcus aureus (1). The prevalence of 4S is higher in infants and children under 5 years of age than in adults (2). Evidence indicates that the incidence of 4S is increasing, especially in developing countries (3, 4). A study in the Czech Republic reported an incidence of 25/1000000 in children under one year old (5, 6). According to a recent study in Iran, the highest prevalence of 4S was observed in Zanjan and Mazandaran provinces, respectively (7).
This syndrome is characterized by the separation of the surface layers of the skin following exposure to the epidermolytic exotoxin produced by S. aureus species (5, 6). After the incubation period, sensitive and painful erythematous rashes develop within 24 to 48 hours. Erythema usually starts in the folds of the body (groin, armpit, neck, and gluteal fold) and progresses to diffuse erythrodermia. Erythrodermia and skin tenderness phases last around 48 hours (6, 8-10). Thin-walled and fragile blisters develop in erythematous areas and are followed by superficial generalized exfoliation. The superficial layers of the epidermis of the skin are separated, and the shiny layer underneath is revealed (5, 6). The spectrum of clinical manifestations can vary from mild local defoliation to the detachment of the surface layer of the skin all over the body, predisposing patients to hypothermia, excessive fluid loss, electrolytes imbalance, and hypovolemic shock, as well as the possibility of additional infections (6, 10, 11).
All patients need to be hospitalized, preferably in isolation in the burn ward or PICU. Supportive care, hydration, temperature monitoring, isolation, and nutrition are critical (6). Antibiotics (covering staphylococci) should be prescribed as soon as possible. Scaling usually occurs within five days, with complete resolution within two weeks, usually without any permanent sequelae. The effective treatment of S. aureus infections largely depends on the type of infection and the presence or absence of drug-resistant strains (12, 13).
There is no consensus on a specific antibiotic regimen for treating 4S (14). Overall, clindamycin, other anti-staphs (MRSA or MSSA), first- and second-generation cephalosporins, and their combinations are used for this purpose (14). There are different ideas about the applicability of clindamycin in the treatment of 4S. It is stated that clindamycin is a preferred and sufficient agent due to its bacterial toxin inhibitory effects (8, 15). However, when using clindamycin, its gastrointestinal side effects should always be taken into consideration. Owing to the increasing prevalence of clindamycin resistance, some guidelines prefer to use this drug as an adjuvant with a penicillinase-resistant penicillin or cephalosporin (8).
2. Objectives
Given the conflicting results on the effectiveness of various treatment strategies, the lack of a single treatment portal, differences in antibiotic resistance patterns, and the possible effect of racial differences on treatment outcomes, this study aimed to compare the effectiveness of clindamycin, clindamycin with another anti-staph agent, and an antibiotic regimen without clindamycin in treating children with 4S. The primary outcome was to compare the efficacy of these different antibiotic regimens in mitigating the clinical course of 4S, and the second goal was to compare the side effects of these therapeutic strategies.
3. Methods
3.1. Study Design and Data Collection
In this cross-sectional study, children with the clinical diagnosis of 4S (based on the final diagnosis recorded by the attending physician in the inpatient medical document) admitted to the 17th-Shahrivar Hospital in Rasht, Iran, from 2005 to 2021 were enrolled. The 17th-Shahrivar Hospital is a specialized and sub-specialized pediatric referral hospital in the Guilan province of Iran. Since the diagnosis of 4S is clinical, we were satisfied with the final diagnosis recorded in the medical file. Eligible patients were identified by searching the electronic database of the hospital.
Exclusion criteria encompassed being a neonate, suffering from chronic skin diseases or immunodeficiencies, and having incomplete hospital data files. Sampling was based on census, and the records of all hospitalized children with the final diagnosis of 4S were inspected. After the approval of the study’s protocol by the research council and ethics committee of the Guilan University of Medical Sciences, a form was prepared to record variables such as age, sex, administered antibiotics, time of fever cessation (if fever existed), time to the first signs of skin lesion healing, duration of hospitalization, and disease- or drug-related complications for each patient. These data were compared between the following three groups: Clindamycin, clindamycin plus another antibiotic, and antibiotic regimens without clindamycin.
3.2. Statistical Analysis
After collecting the data, they were entered into SPSS v.24 software. In order to determine the relationship between the therapeutic efficacy of clindamycin and the variables investigated in this study, either the ANOVA (if the distribution of quantitative variables was normal) or Kruskal-Wallis (if the data distribution was non-normal) test was employed. The LSD post-hoc test was used to designate the significant difference between the two groups. For better understanding, we defined the following three options for post-hoc assessments:
Option 1: Clindamycin alone versus clindamycin plus other antibiotics
Option 2: Clindamycin plus other antibiotic versus antibiotic regimens without clindamycin
Option 3: Clindamycin alone versus antibiotic regimens without clindamycin
The chi-square test was used to assess the relationship between qualitative variables. The data were analyzed using SPSS version 24 software. Quantitative variables were shown as "mean ± standard deviation", and qualitative variables were displayed as "frequency (percentage)". The Kolmogorov-Smirnov test was used to check the normality of quantitative variables, and a P-value less than 0.05 was considered statistically significant.
3.3. Ethical Considerations
Ethical approval was obtained from the Research Ethics Committee of Guilan University of Medical Sciences (code: IR.GUMS.REC.1400.612, date: 2022-03-09).
4. Results
This study was conducted on 73 children with the final clinical diagnosis of 4S admitted to our hospital in the specified time period. The mean age of the patients was 17.70 ± 15.85 months; the minimum age was one month, and the maximum age was 89 months. Most of the children (n = 43, 58.9%) were male. In this study, the mean body temperature was 37.62 ± 0.73°C (the lowest and highest: 36.70 and 39.50°C, respectively). Thirty-five children (47.9%) had a fever, and 38 children (52.1%) did not experience a fever during hospitalization. The average duration of fever was 16.20 ± 9.89 hours, with the minimum and the maximum durations of 4.00 and 54.00 hours, respectively.
Most children (n = 42, 57.5%) were treated with clindamycin in combination with another antibiotic. Eleven patients (15.1%) received clindamycin alone, and 20 children (27.4%) were treated with antibiotics other than clindamycin, including vancomycin, cloxacillin, and ceftriaxone).
The mean duration of hospital stay was 6.52 ± 1.90 days (minimum: 3 and maximum: 13 days). Also, the results showed that the average duration of recovery in these children was 4.90 ± 1.73 days (minimum: 2 and maximum: 11 days).
Comparison of the antibiotics received based on sex (using the chi-square test) and age (using the ANOVA test) indicated that boys constituted most of the children in all three treatment groups with no statistically significant difference (P-value = 0.245). Also, there was no significant difference in the mean age of patients between the three therapeutic groups (P-value= 0.383) (Table 1).
| Variables | Type of Administered Antibiotic | P-Value | ||
|---|---|---|---|---|
| Antibiotics Other than Clindamycin | Clindamycin Alone | Clindamycin + Another Anti-S. aureus Antibiotic | ||
| Male (female) | 9 (2) | 11 (9) | 23 (19) | 0.245 |
| Age (month) | 20.55 ± 24.63 | 20.80 ± 18.40 | 15.48 ± 11.15 | 0.383 |
a Values are expressed as mean ± SD unless otherwise indicated.
Comparing the type of antibiotics used based on the presence of fever indicated that febrile children were mostly treated with clindamycin plus another antibiotic than with other treatment strategies (P-value = 0.008, Table 2).
| Fever | Type of Administered Antibiotics | P-Value | ||
|---|---|---|---|---|
| Antibiotics Other than Clindamycin | Clindamycin Alone | Clindamycin + Another anti-S. aureus Antibiotic | ||
| Present | 5 (45) | 4 (20) | 26 (61) | 0.008 |
| Absent | 6 (54) | 16 (80) | 16 (38) | |
| Total | 11 (100) | 20 (100) | 42 (100) | |
a Values are expressed as No. (%).
The results of the ANOVA test showed that the duration of fever was comparable between the three treatment groups (P-value = 0.568). The LSD post-hoc test showed that there was no significant difference between the three groups regarding options 1, 2, and 3 (P-value = 0.434, 0.4124, and 0.976, respectively). Meanwhile, a significant difference was observed between the three groups in terms of the length of the recovery time and hospitalization period. The recovery (P-value = 0.018) and hospitalization (P-value = 0.002) periods were significantly longer in the group receiving no clindamycin (Table 3). Regarding the duration of recovery, the results showed no significant difference in option 1 (P-value = 0.367) but significant differences for options 2 (P-value = 0.017) and 3 (P-value = 0.006). In terms of the hospitalization period, no significant difference was observed for option 2 (P-value = 0.159). However, significant differences were noticed for options 1 (P-value = 0.044) and 3 (P-value = 0.007), indicating the shorter duration of hospitalization in the patients who received clindamycin alone compared to those receiving clindamycin plus another antibiotic (P-value = 0.044).
| Variables | Treatment Groups | P-Value | ||
|---|---|---|---|---|
| Clindamycin + Another anti-S. aureus Antibiotic | Clindamycin Alone | Antibiotics Other than Clindamycin | ||
| Duration of fever (h) | 17.6 ± 10.68 | 8.24 ± 13.00 | 13.20 ± 6.26 | 0.568 |
| Recovery time (day) | 4.80 ± 1.59 | 4.40 ± 1.81 | 7.54 ± 2.46 | 0.018 |
| Hospitalization length (day) | 6.66 ± 1.63 | 5.65 ± 1.81 | 7.54 ± 2.46 | 0.020 |
a Values are expressed as mean ± SD.
Regarding disease-related complications, 13 patients had no complications, and the remaining 60 patients reported 93 complications. The most common finding among patients was scaling, which was reported alone or with other symptoms in 42 cases. Isolated scaling was present in 21 patients (Table 4).
| Complications | No. (%) |
|---|---|
| Scaling | 42 (45.16) |
| Diarrhea | 16 (17.2) |
| Vomiting | 13 (13.97) |
| Eosinophilia | 7 (7.50) |
| Liver enzymes elevation | 6 (6.45) |
| Neutropenia | 6 (6.45) |
| Leukocytosis | 1 (1.07) |
| Thrombocytosis | 1 (1.07) |
| Itching | 1 (1.07) |
| Total | 93 (100) |
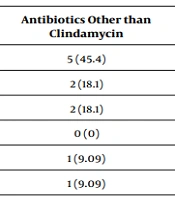
As shown in Table 5, there was no significant difference in the occurrence of disease/drug-related complications between the three groups, and the most common finding in all three groups was scaling (P-value = 0.412).
| Complications | Treatment Groups | P-Value | ||
|---|---|---|---|---|
| Clindamycin + Another Anti-S. aureus Antibiotic | Clindamycin Alone | Antibiotics Other than Clindamycin | ||
| Scaling | 23 (43.3) | 14 (48.2) | 5 (45.4) | 0.412 |
| Diarrhea | 9 (16.9) | 5 (17.2) | 2 (18.1) | |
| Vomiting | 8 (15.09) | 3 (10.3) | 2 (18.1) | |
| Eosinophilia | 5 (9.4) | 2 (6.89) | 0 (0) | |
| Liver enzymes elevation | 2 (0.3) | 3 (10.3) | 1 (9.09) | |
| Neutropenia | 5 (9.4) | 0 (0) | 1 (9.09) | |
| Leukocytosis | 0 (0) | 1 (3.4) | 0 (0) | |
| Thrombocytosis | 1 (1.8) | 0 (0) | 0 (0) | |
| Itching | 0 (0) | 1 (3.4) | 0 (0) | |
| Total | 53 (100) | 29 (100) | 11 (100) | |
a Values are expressed as No. (%).
5. Discussion
According to past experiences, clindamycin, either alone or in combination with another anti-staphylococcal agent, is one of the antibiotics generally recommended for treating 4S (14, 16). The present study was conducted to evaluate the efficacy of different antibiotic regimens, with or without clindamycin, in the treatment of this syndrome in children. Overall, our results showed that treatment regimens containing clindamycin (alone or in combination with other agents) were more frequently used by practitioners in our hospital to treat 4S, especially in feverish children. On the other hand, these results showed that clindamycin (alone or in combination) could reduce the duration of the disease and the length of hospitalization.
Monotherapy by clindamycin, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most common anti-staphylococcal antibiotic regimens recommended for children admitted with 4S to pediatric hospitals in the United States (14). Also, European protocols suggest clindamycin in combination with other anti-staphylococcal agents as the accepted therapeutic approach for treating 4S (17). In a populous country such as China, there is also a similar therapeutic approach (18). Evidence has shown that clindamycin has excellent skin penetration and can reduce the production of staphylococcal exotoxins, which is one of the important factors in the pathogenesis of 4S (10). In this regard, Mahmoudi and colleagues also found that clindamycin could significantly improve the clinical course of 4S patients (19).
Clindamycin has a good potential for treating S. aureus infections in children, but increasing evidence indicates the occurrence of drug resistance in recent decades (8, 19, 20). In this regard, most global guidelines emphasize the combined administration of anti-staph drugs with clindamycin to achieve better therapeutic effectiveness (19, 20).
The current research showed that the most common complication during the course of 4S was scaling. Davey et al., in a case report, described that the use of clindamycin could induce skin shedding and toxic epidermal necrosis (21). On the other hand, desquamation is also a common finding in the clinical course of 4S (22). Whether scaling is caused by the disease itself or it is a drug side effect cannot really be proven, and it is challenging to consider this finding a side effect of antibiotic therapy (23).
Studies have shown that exposure to antibiotics is a risk factor for developing diarrhea, especially when 2 or more antibiotics are used together (24, 25). Also, diarrhea caused by Clostridium difficile is one of the known side effects of clindamycin treatment (25, 26). In the present study, gastrointestinal complications, especially diarrhea, were reported as side effects, but there was no significant difference in their occurrence between patients who received or not received clindamycin.
In this research, evidence showed that the administration of clindamycin (alone or in combination with other antibiotics) could not significantly reduce the duration of fever compared to regimens containing antibiotics other than clindamycin. Levison et al. compared the efficacy of clindamycin and penicillin and observed that clindamycin had better therapeutic and anti-fever effects in patients with bacterial infections (27), which was in contrast to our findings. It should be noted that the recent study was conducted in the 1980s, so this difference can be justified by the occurrence of bacterial resistance in recent decades. On the other hand, McKeown and Baker also achieved results similar to ours, reporting that clindamycin and other antibiotics performed equally in reducing the duration of fever in neonates diagnosed with 4S (28).
Clindamycin resistance is rising in different strains of S. aureus (29), including in strains causing 4S (30). Vernali et al. found that clindamycin resistance was more common in hospitalized 4S patients compared to pediatric outpatients. Evidence shows that the combination of clindamycin with other antibiotics can tackle staphylococcal resistance, expressing promising findings for the combination of vancomycin and clindamycin (31). It should be mentioned that in our study, most patients with fever at admission received clindamycin plus another antibiotic.
In the present study, the use of clindamycin (either in combination with another antibiotic or alone) reduced the duration of recovery, as well as hospitalization length, in children with 4S. In this regard, Kosior and Reich found that clindamycin, in combination with other antibiotics, reduced the length of hospitalization in patients with various infections caused by S. aureus (32), which was consistent with the present study. It is worth noting that the recommended agent in the recent study also included co-amoxiclav, and in this regard, it shows some differences compared to our findings. In addition, Tissot-Dupont et al. declared that the combination of high-dose clindamycin with other anti-staph drugs could inhibit life-threatening infections, such as endocarditis, caused by S. aureus and reduce the length of hospitalization, indicating the acceptable capacity of this antibiotic for treating various infections (33). However, Neubauer et al. found no significant difference in terms of the therapeutic response between the patients receiving clindamycin alone and those treated with clindamycin + other anti-staph antibiotics (MSSA or MRSA) (14), which may be justified by climatic differences and the occurrence of more resistance in the USA. Neubauer et al. further concluded that the addition of anti-MSSA or MRSA agents to clindamycin was associated with increased costs with no incremental differences in the clinical outcomes of pediatric 4S (14). On the other hand, in their study, all three groups received clindamycin; however, in the present study, the therapeutic response was also investigated in patients receiving antibiotics other than clindamycin. In a retrospective cohort study by Gray et al., they encountered 36% resistance to clindamycin and ruled out that clindamycin could improve patient outcomes, which was contrary to our results. The authors suggested beta-lactams as the first therapeutic line (34). In this regard, Liy-Wong et al. concluded that the addition of clindamycin to other anti-staph antibiotics had no beneficial effect on the duration of hospitalization (9). Yang et al. noted that resistance rates to levofloxacin (8.33%), gentamycin (8.33%), tetracycline (25%), oxacillin (8.33%), and vancomycin (0%) were significantly lower than that to erythromycin (100%), trimethoprim-sulfamethoxazole (TMP/SMX) (83.33%), clindamycin (91.67%), and penicillin G (100%) in 4S patients, showing that vancomycin and oxacillin had the lowest resistance rates. Overall, resistance to clindamycin was high in the report of Yang et al., causing them to pose against clindamycin monotherapy for treating 4S (15). A retrospective study by Braunstein et al. suggested that oxacillin-susceptible and clindamycin-resistant strains of S. aureus were predominantly associated with 4S (35). Buchwald et al. found that higher doses of clindamycin were not superior to other lower doses in terms of their impact on the duration of treatment and length of hospitalization (36).
In the present study, a higher percentage of febrile children were treated with clindamycin than other anti-staph agents. In addition, evidence indicated that despite the presence of systemic symptoms (before treatment) in a higher ratio of these patients, they enjoyed a faster recovery period than their counterparts. Despite no difference in terms of the duration of fever and recovery, the present study showed that the patients who received clindamycin alone had a shorter hospital stay than patients who were treated with clindamycin plus other antibiotics. Finally, among the three different therapeutic options, considering the possible complications and higher costs of dual therapy, our findings favor clindamycin monotherapy for treating children with 4S regarding its association with a shorter length of hospitalization.
Limitation: We could not design a cohort study, which was one of the limitations of our research due to the small number of 4S patients referred within one year. Certainly, a prospective cohort study can eliminate the effects of patients’ general conditions on the selection of treatments, so one can recruit the same number of patients in different therapeutic groups.
5.1. Conclusions
Clindamycin (alone or in combination with other anti-staphylococcal agents) could shorten the recovery and hospitalization periods in children with 4S without having any adverse impact on the occurrence of disease- or treatment-related complications. Also, we showed that the patients who received clindamycin alone had a shorter hospital stay than their peers treated with clindamycin plus another antibiotic. Considering the lower rate of complications in clindamycin monotherapy, as well as its lower costs and superior effects on shortening the length of hospital stay, we recommend using clindamycin alone to treat 4S patients.