1. Background
Sepsis is one of the important causes of morbidity and mortality in newborns (1, 2). In the world, neonatal sepsis is reported in three million cases per year (3), which has many risk factors. Group B Streptococcus (GBS) and E. coli are the most common bacterial causes, and herpes simplex virus, enterovirus, parvovirus, and Candida are non-bacterial causes of early and late sepsis (4, 5). Maternal factors associated with the risk of early neonatal sepsis include chorioamnionitis, maternal fever during labor, maternal GBS colonization, preterm delivery, and prolonged rupture of membranes (1, 6).
Moreover, neonates with metabolic factors, including hypoxia, acidosis, hypothermia, and hereditary metabolic disorders such as galactosemia, are likely to be at higher risk and more severe neonatal sepsis, which can disrupt the infant's immunological response (7). Infants with sepsis show non-specific signs and symptoms such as temperature instability, irritability, lethargy, tachypnea, grunting, hypoxia, poor feeding, tachycardia, poor perfusion, and hypotension (7). Therefore, the laboratory examination of these infants is very important for timely diagnosis and treatment (1, 7).
In these newborns, diagnostic evaluation includes blood culture, lumbar puncture, Complete Blood Count (CBC), chest radiography if needed, and secretions culture. However, the levels of C-reactive protein (CRP) and/or procalcitonin (PCT) are more important to follow the treatment response and determine the treatment duration through consecutive measurements (7). Resistant pathogens are the cause of 214 000 neonatal deaths annually, and the emergence of multidrug-resistant bacteria imposes challenges in treating neonatal sepsis (8).
In this regard, gentamicin with either ampicillin or benzylpenicillin is recommended as the first-line treatment of neonatal sepsis in resource-limited settings by the World Health Organization (WHO), and third-generation cephalosporins are recommended as second-line therapy (9). Time of infection and geographical location are two important factors associated with the most frequent organisms (8). Recent systematic reviews of antimicrobial resistance (AMR) in neonates and children in sub-Saharan Africa have shown the increasing prevalence of antibiotic resistance, especially in Gram-negative bacteria (10). In South Asia, gram-negative pathogens are predominant, but the prevalence of GBS is low in contrast to high-income countries (11). In a study conducted in Tehran involving 84 neonates, Enterobacter species emerged as the predominant pathogen, with a higher prevalence observed in cases of early sepsis (12).
Similarly, a study carried out in Tabriz, Iran, identified coagulase-negative Staphylococcus and Staphylococcus aureus as the primary pathogens isolated from the blood of neonates with sepsis (13). Moreover, a systematic review and meta-analysis conducted in Iran revealed an overall neonatal sepsis prevalence of 16%, predominantly affecting male neonates. Among the identified pathogens, Enterobacter and Klebsiella pneumoniae were most frequently detected (14).
2. Objectives
Since information on the bacteriological profile and associated antimicrobial sensitivity for its treatment are essential to combat neonatal morbidity and mortality, this study aimed to investigate the bacterial profile and antibiotic sensitivity in neonates with sepsis.
3. Methods
In this descriptive-analytical cross-sectional study, all neonates with sepsis proven by blood culture who were admitted to the Neonatal Intensive Care Unit (NICU) of Namazi Hospital, Shiraz, Iran, from 2020 to 2021 were studied. Based on a systematic review in Iran that reported the prevalence of neonatal sepsis as 14.3%, taking into account the confidence level of 95%, the margin of error of 5%, and d value of 20% using the formula
Blood culture was requested for all neonates suspected of sepsis. It needs at least 1 ml of blood. Inclusion criteria consisted of all neonates with sepsis who had positive blood cultures. We considered neonates with blood cultures of Staphylococcus epidermidis as sepsis in the presence of at least one of the clinical signs of sepsis, a rise in CRP, and a detection time of less than 36 hours to rule out the possibility of contamination. Neonates who had positive blood cultures but incomplete medical records were excluded.
Demographic and medical information including GA, gender, age of onset of sepsis, birth weight, history of previous disease, history of the maternal underlying disease and infections, history of premature rupture of the membrane and its duration, delivery method, and duration of hospitalization were collected by reviewing patients' records.
Other information, including clinical symptoms (fever, poor feeding, and lethargy), laboratory results (CBC and CRP), results of blood culture, urine analysis, urine culture, and antibiogram were also recorded in a researcher-made checklist.
The normal range was 5000 - 20000 cells/µL for WBC count, 35 - 60% for neutrophils, and 25 - 55% for lymphocytes. Hemoglobin was considered normal within the range of 13.45 - 22 g/dl and platelet within 150 000 to 450,000 per microliter. Neonates born before 37 weeks of GA were considered preterm, and those born with GA ≥ 37 weeks were considered term neonates. Early sepsis is defined when it occurs at or before 72 hours of life, and late-onset sepsis occurs after the first 72 hours.
3.1. Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 21.0 software (SPSS Inc., Chicago, IL, USA). The quantitative variables were reported by the mean, Standard Deviation (SD) [95% Confidence Interval (CI)], and qualitative ones were reported by frequency (%).
We used the Kolmogorov-Smirnov test to check the normal distribution of data. Data were analyzed by a two-independent sample t-test, Mann-Whitney U, Chi-square, Pearson's correlation coefficient, Spearman's rank correlation coefficient, Analysis of Variance (ANOVA), and Kruskal Wallis tests. A P-value of less than 0.05 was considered statistically significant. The ethical code for this project is IR.SUMS.MED.REC.1400.454.
4. Results
This study showed that among 90 sepsis patients with an average age of 6.7 ± 7.6 days, 54 (60.0%) were male and 36 (40.0%) were female. Also, 58 (64.4%) were preterm, and 32 (35.6%) were full-term. Fifty (55.6%) neonates had an Apgar score of less than 7 in the first minute of birth, and 26 (28.9%) had an Apgar score of less than 7 in the fifth minute.
Among 50 (55.5%) neonates who had early sepsis, 39 (78%) were preterm, and 11 (22%) were full-term. Forty neonates (44.4%) had late sepsis: 19 (47.5%) preterm and 21 (52.5%) term. Other demographic data are shown in Table 1.
| Variables | Values |
|---|---|
| Age (days) | 6.7 ± 7.4 |
| Early sepsis | 1.29 ± 0.68 |
| Late sepsis | 12.85 ± 6.7 |
| Age at birth (w) | 34.14 ± 3.75 |
| Early sepsis | 33.04 ± 3.85 |
| Late sepsis | 35.40 ± 3.24 |
| Birth weight (g) | 2345.28 ± 881.42 |
| Early sepsis | 2076.15 ± 828.16 |
| Late sepsis | 2652.86 ± 847.65 |
| Age of onset of disease symptoms (days) | 6.07 ± 6.93 |
| Early sepsis | 1.35 ± 0.72 |
| Late sepsis | 11.47 ± 6.91 |
| Duration of hospitalization (days) | 28.66 ± 18.4 |
| 1-minute Apgar | 6.84 ± 1.73 |
| 5-minute Apgar | 8.19 ± 1.44 |
| Gender | |
| Female | 36 (40) |
| Male | 54 (60) |
| Delivery type | |
| Vaginal delivery | 47 (52.2) |
| Cesarean section | 41 (45.6) |
| Type of feeding | |
| Breastfeeding | 53 (58.9) |
| Milk bottle | 36 (40.4) |
| Rupture of membrane history (> 18 h) | 17 (18.9) |
| History of herbal medicine using | 8 (8.9) |
| Fever in newborn | 24 (26.7) |
| Lethargy | 45 (50) |
| Poor feeding | 46 (51.1) |
| Seizure | 12 (13.3) |
a Values are presented as No. (%) ± or mean SD.
The analysis of CBC revealed that 77 (85.6%) of the neonates had normal WBC counts, 44 (49.4%) had normal neutrophil counts, 36 (40.4%) exhibited neutropenia, and 9 (10.2%) had elevated neutrophil levels. Additionally, 32 (35.9%) neonates had normal lymphocyte counts, 7 (7.8%) had lymphopenia, and 50 (55.2%) had lymphocytosis. Hemoglobin levels fell within the normal range for 52 (57.8%) neonates, and platelet counts were normal in 56 (62.2%) of the cases.
In this study, the highest frequency was related to Klebsiella, accounting for 22 (24.4%) positive blood cultures. Also, the lowest frequency was related to Non-fermenting bacilli, which was observed in a one-day-old, preterm male neonate with a hospitalization duration of 15 days. Other microorganisms observed were S. epidermidis (18.9%), Enterococcus (16.7%), Acinetobacter (11.1%), Stenotrophomonas maltophilia (9%), E. coli (5.6%), Serratia and Pseudomonas aeruginosa (4.4%), and S. aureus (3.3%). Gram-negative organisms were grown in 61% of positive blood cultures, the most common of which was Klebsiella pneumonia, while among Gram-positive organisms, S. epidermidis was the most common (Table 2).
| Values | No. (%) |
|---|---|
| Microorganism | |
| Staphylococcus epidermidis | 17 (18.9) |
| Serratia | 4 (4.4) |
| Acinetobacter | 10 (11.1) |
| Stenotrophomonas maltophilia | 9 (10) |
| Klebsiella | 22 (24.4) |
| Enterococcus | 15 (16.7) |
| Staphylococcus aureus | 3 (3.3) |
| Pseudomonas aeruginosa | 4 (4.4) |
| Non-fermenting bacilli | 1 (1.1) |
| E. coli | 5 (5.6) |
| Total | 90 (100) |
In this study, the significant difference in microbial composition in preterm and term infants was evaluated. The statistical analysis revealed no significant difference in E. coli (P-value 0.32). Similarly, no significant difference was observed in Klebsiella (P-value 0.19). Staphylococcus epidermidis and Enterococcus also exhibited no statistically significant variations, with P-values of 0.98 and 0.84, respectively.
In premature babies under 34 weeks, the frequency of early sepsis was 73.8%, and the most common organisms were Klebsiella (35.5%) and S. epidermis (22.5%). Meanwhile, other gram-negative organisms included Pseudomonas aeruginosa, E. coli, Acinetobacter, Serratia, and Stenotrophomonas maltophilia. The frequency of late sepsis in this group of patients was 26.2%; the highest frequency was related to S. epidermis (27%); other organisms included Klebsiella, Enterococcus, and Acinetobacter with the same frequency.
In late preterm newborns (gestational age between 34 and 36 weeks), the incidence of early and late-onset sepsis was 58.8% and 41.2%, respectively. The most common organism in early-onset sepsis and late-onset sepsis was Klebsiella.
In term neonates over 37 weeks, the incidence of early-onset sepsis (39%) was lower than that of late-onset sepsis (61%). The most common microorganisms in the two groups were Acinetobacter, Enterococcus, and S. epidermises. Our results showed that the incidence of early-onset sepsis decreased with increasing gestational age.
Most organisms were sensitive to colistin (37 neonates), and the highest resistance rate was to cefotaxime (35 neonates). The more susceptibility of gram-positive organisms was to vancomycin, imipenem, meropenem, and linezolid, while gram-negative organisms were mainly resistant to aminoglycosides and cefotaxime, which are widely used in the NICUs (Table 3). The sensitivity and resistance of effective antibiotics in sepsis on gram-negative organisms are shown in Table 4.
| Antibiotics | Gram-Positive Organisms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus Epidermis (N = 17) | Enterococcus (N = 15) | Staphylococcus aureus (N = 3) | |||||||
| S | R | T | S | R | T | S | R | T | |
| Ampicillin | 0 | 0 | 0 | 0 | 3 (100) | 3 | 0 | 0 | 0 |
| Clindamycin | 2 (33.3) | 6 (66.7) | 8 | 0 | 5 (100) | 5 | 0 | 2 (100) | 2 |
| Cloxacillin | 1 (100) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imipenem | 3 (100) | 0 | 3 | 2 | 1 | 3 | 0 | 0 | 0 |
| Linezolid | 1 (100) | 0 | 1 | 0 | 0 | 0 | 2 (100) | 0 | 0 |
| Meropenem | 0 | 0 | 0 | 5 (100) | 0 | 5 | 0 | 0 | 0 |
| Penicillin | 0 | 1 (100) | 1 | 1 (100) | 0 | 1 | 0 | 0 | 0 |
| Rifampin | 1 (100) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vancomycin | 15 (100) | 0 | 15 | 3 (100) | 0 | 3 | 2 (100) | 0 | 2 |
| Gentamicin | 1 (10) | 9 (90) | 10 | 1 (25) | 3 (75) | 4 | 0 | 1 (100) | 1 |
| Ciprofloxacin | 2 (16.7) | 10 (83.3) | 12 | 4 (36.3) | 7 (63.7) | 11 | 1 (100) | 0 | 1 |
Abbreviations: T, total; R, resistance; S, sensitivity.
| Antibiotics | Gram-Negative Organisms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Klebsiella (N = 22) | Acinetobacter (N = 10) | Stenotrophomonas maltophilia (N = 9) | |||||||
| S | R | T | S | R | T | S | R | T | |
| Amikacin | 1 (10) | 9 (90) | 10 | 1 (12.5) | 7 (87.5) | 8 | 3 (37.5) | 5 (62.5) | 8 |
| Gentamicin | 3 (42.8) | 4 (57.2) | 7 | 1 (50) | 1 (50) | 2 | 2 (100) | 0 | 2 |
| Meropenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 1 |
| Imipenem | 4 (66.7) | 2 (33.3) | 6 | 1 (16.7) | 5 (83.3) | 6 | 0 | 8 (100) | 8 |
| Cefotaxime | 1 (5.9) | 16 (94.1) | 17 | 0 | 1 (100) | 1 | 0 | 2 (100) | 2 |
| Ciprofloxacin | 10 (83.3) | 2 (16.7) | 12 | 0 | 2 (100) | 2 | 4 (80) | 1 (20) | 5 |
| Colistin | 16 (100) | 0 | 16 | 7 (100) | 0 | 7 | 3 (100) | 0 | 3 |
| Ceftazidime | 0 | 1 (100) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefazolin | 0 | 7 (100) | 7 | 0 | 1 (100) | 1 | 0 | 2 (100) | 2 |
| Cefalotin | 0 | 3 (100) | 3 | 0 | 2 (100) | 2 | 0 | 0 | 0 |
Abbreviations: T, total; R, resistance; S, sensitivity.
There was a significant difference in the age of onset of sepsis symptoms between term (8.41 ± 7.39) and premature infants (4.8 ± 6.37) (P = 0.002). Nevertheless, the average length of hospitalization was not significantly different between full-term (26.17 ± 47.84) and premature infants (29.18 ± 86.71) (P = 0.3). Also, there was no relationship between the duration of hospitalization and the type of microorganism causing sepsis (P = 0.4).
Based on the results, there was a significant relationship between the age of onset of symptoms and birth weight in neonates with positive blood cultures (P = 0.004) (r = 0.3). The results showed that the lower the birth weight, the lower the age of onset of sepsis. However, the duration of hospitalization and the type of microorganism causing sepsis was not related to birth weight (P = 0.35 and P = 0.82, respectively). Moreover, there was no significant difference in the mean age of onset of sepsis symptoms between the two groups with and without a history of PROM (P = 0.31). In this regard, statistical tests showed a significant relationship between clinical manifestations, such as fever, lethargy, reduced breastfeeding, tachypnea, retraction, nasal flaring, and birth weight and gestational age (term and preterm) (P < 0.05) (Table 5).
| Variables | Birth Weight | P b | Gestational Age | P c | |
|---|---|---|---|---|---|
| Preterm (< 37 week) | Term (≥ 37 week) | ||||
| Fever | 0.015 | 0.006 | |||
| Yes | 2717.29 ± 832.30 | 10 (17.2) | 14 (43.8) | ||
| No | 2210 ± 865.42 | 48 (82.8) | 18 (56.3) | ||
| Lethargy | 0.007 | 0.002 | |||
| Yes | 2593.67 ± 854.78 | 22 (37.9) | 23 (71.9) | ||
| No | 2096.89 ± 854.34 | 36 (62.1) | 9 (28.1) | ||
| Poor feeding | 0.032 | 0.024 | |||
| Yes | 2539.04 ± 880.73 | 25 (43.9) | 22 (68.8) | ||
| No | 2136.79 ± 851.83 | 32 (56.1) | 10 (31.3) | ||
| Seizure | 0.193 | 0.16 | |||
| Yes | 2670.91 ± 846.78 | 5 (8.6) | 6 (18.8) | ||
| No | 2299.94 ± 881.76 | 53 (91.4) | 26 (81.3) | ||
| Tachypnea | 0.006 | 0.002 | |||
| Yes | 2164.09 ± 838.99 | 42 (73.7) | 13 (40.6) | ||
| No | 2680.88 ± 842.24 | 15 (26.3) | 19 (59.4) | ||
| Retraction | < 0.001 | < 0.001 | |||
| Yes | 2040.91 ± 794.99 | 45 (77.6) | 10 (31.3) | ||
| No | 2823.57 ± 802.82 | 13 (22.4) | 22 (68.8) | ||
| Nasal flaring | 0.001 | < 0.001 | |||
| Yes | 1986.22 ± 767.27 | 32 (55.2) | 5 (15.6) | ||
| No | 2595.94 ± 875.42 | 26 (44.8) | 27 (84.4) | ||
| Abdominal distension | 0.612 | 0.242 | |||
| Yes | 2452.37 ± 878.16 | 10 (17.5) | 9 (28.1) | ||
| No | 2336.86 ± 876.02 | 47 (82.5) | 23(71.9) | ||
a Values are presented as No. (%) or mean ± SD.
b Independent t-test.
c Chi-square test. P < 0.05 is considered significant.
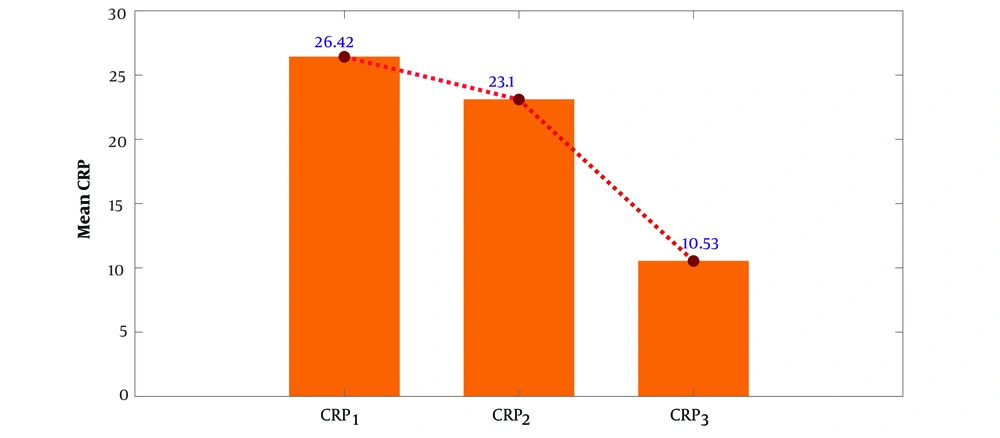
Following the treatment, the CRP value showed a decreasing trend in three periods (Figure 1), and there was no significant relationship between CRP values and birth weight (P = 0.56), the length of hospitalization (P = 0.94), and the age of the neonates (P = 0.55). The noteworthy point is that in 60% of neonates, the first CRP was negative, which then increased in the subsequent measurement.
5. Discussion
The study investigated the bacterial profile and antibiotic sensitivity in infants with sepsis hospitalized in the NICUs of Namazi Hospital in Shiraz. The results showed that the prevalence of sepsis was higher in boys than in girls. In a systematic analysis and meta-analysis, Akbarian-Rad et al. showed that the prevalence of sepsis was slightly higher in boys than in girls (14). Also, in the study of Aku et al., 60.7% of patients were male (15). Therefore, the results of these studies are consistent with our results, and the male gender can be considered a possible risk factor for neonatal sepsis.
Neonatal sepsis is classified into early-onset, occurring within the first 72 hours, and late-onset, manifesting after 72 hours. Our study found that the majority of neonatal sepsis cases (55.5%) occurred within the initial 72 hours. In line with these findings, a systematic review of neonatal sepsis in Iran reported a pooled prevalence of 10.96 for early-onset sepsis, with a recorded prevalence of 411 cases out of 8025 neonates. Furthermore, the review documented a pooled interval of 6.85 for late-onset sepsis, observed in 216 out of 8025 neonates (14). Notably, a significant proportion of neonates affected by early-onset sepsis were preterm infants (78%). Klebsiella and S. epidermidis were the most prevalent among the pathogens identified. Our study revealed that most cases of late-onset sepsis were observed among term neonates. According to the American Neonatology Network, gram-positive microorganisms were responsible for 62% of early-onset sepsis, while in late sepsis, gram-positive microorganisms accounted for 75% of the cases (16, 17).
Based on our findings, more than 60% of blood cultures were positive with gram-negative organisms, the highest frequency of which was K. pneumonia. Among the gram-positive organisms, S. epidermidis was the most frequent. Also, the incidence of early-onset sepsis decreased with increasing gestational age. In this regard, another study in Iran by Akya et al. (18) showed that the most common bacteria causing neonatal sepsis were K. pneumonia and P. aeruginosa (gram-negative), coagulase-negative Staphylococcus, and S. aureus (gram-positive). Also, Akbarian-Rad et al. showed that the most common causative bacterial pathogens were Enterobacter species (23.04%), K. pneumonia (17.54%), coagulase-negative Staphylococcus (14.06%), E. coli (13.92%), P. aeruginosa (12.67%), and S. aureus (11.8%), in sequence (14). Therefore, gram-negative bacteria are the most common cause of neonatal sepsis in Iran (18). Alharbi in Saudi Arabia revealed that coagulase-negative Staphylococcus and Klebsiella species were the most common microorganisms (57.5%) in blood cultures (19). However, in the study by Li et al., gram-positive bacteria were reported as the primary causative agent of neonatal septicemia in their hospital, among which S. epidermidis (47.17%) was the primary conditional pathogen. Also, E. coli (49.09%) was mainly isolated among gram-negative pathogens (20). Furthermore, Peterside et al. reported that gram-positive organisms (53.6%) were more frequent than gram-negative organisms (46.4%). The most common organism isolated was S. aureus (51.5%), followed by E. coli (16.5%) and K. pneumonia (14.4%) (21). These variations in findings between regions could be attributed to factors such as local epidemiological differences, healthcare practices, and the prevalence of certain bacterial strains. Nonetheless, the overall consensus appears to be that both gram-positive and gram-negative organisms can be significant contributors to neonatal sepsis, with the specific distribution varying by location.
Our findings showed that most organisms were sensitive to colistin and resistant to cefotaxime. In the present study, gram-positive organisms were most susceptible to vancomycin, imipenem, meropenem, and linezolid, and gram-negative microorganisms were mainly resistant to aminoglycosides and cefotaxime. In Li et al.'s study, gram-positive bacteria were more sensitive to vancomycin, tigecycline, and linezolid, and E. coli was 100% susceptible to piperacillin, cefotetan, meropenem, and imipenem (20). In the study of Stoll et al., most of the isolated microorganisms showed high sensitivity to ampicillin and gentamicin (16). Another survey by Pokhrel et al. also showed that mainly Klebsiella species had increased resistance to common antibiotics, including cefotaxime, gentamicin, ciprofloxacin, ofloxacin, and chloramphenicol. However, they showed good sensitivity to carbapenems, colistin, and tigecycline. Besides, CONS had high resistance to oxacillin, cefotaxime, and meropenem and 100% sensitivity to vancomycin and linezolid (22). Overall, these findings and the literature review emphasize the importance of antibiotic stewardship and the need for healthcare providers to select antibiotics carefully based on local resistance patterns to optimize treatment for neonatal sepsis.
In our study, the first CRP was negative in 60% of neonates, and then, in the subsequent measurements, we observed a rise in the level of CRP. So, CRP is an important factor in evaluating the treatment process. Also, CRP is considered a valuable marker with a sensitivity of 87.5% and a specificity of 90.5% in neonatal septicemia (23). Thus, CRP estimation can help in identifying neonatal sepsis, but it is not accurate enough to be the sole criterion for diagnosis (24).
This study provides valuable region-specific data, aiding in better understanding and management of neonatal sepsis and shed light on effective antibiotic choices based on sensitivity profiles, potentially improving treatment outcomes for affected neonates.
5.1. Study Limitations
The limitations of this study included the relatively small number of patients previously treated with antibiotics, in addition to the fact that it was a single-center study in a short period. Therefore, more extended study periods are recommended. The antibiotics used for each antibiogram were also different from each other, which could influence the results of our study. Local protocols are needed for each NICU based on the specific microorganisms and antimicrobial pattern. Multicenter data collection and analysis of antibiotic resistance emergence are suggested to help develop local and national protocols for better outcomes.
5.2. Conclusions
Our study revealed that gram-negative bacteria stand out as the predominant culprits behind sepsis, mainly resistant to aminoglycosides and cefotaxime. Therefore, increasing awareness about the optimal use of antibiotics is necessary to curb the increase in resistance levels. Further investigation and more studies are needed to develop local and international guidelines based on the most frequently isolated organisms and antimicrobial sensitivity.