1. Background
Pertussis (whooping cough) with substantial morbidity and mortality is caused by gram-negative pleomorphic bacillus Bordetella pertussis (1). The highest mortality rate is observed among infants < 6 months of age (2).
In spite of high vaccine coverage, high incidence of pertussis and its mortality rate have been reported from many countries, such as England, Wales, the United States, Portugal, and New Zealand, during the recent years (3). In the United States, the annual incidence of pertussis has increased by 3 folds since 1980 with immunization rates of 80% (2, 3). The reasons for the ongoing outbreaks in such countries with high immunization rates are unknown and multiple factors seem to be involved (4).
Pregnant females have low concentrations of pertussis antibodies to transfer and immune their fetuses (5). Moreover, vaccination against pertussis is licensed for infants after 6 weeks of age while the first dose provides only brief protection (6).
Studies have shown that like maternal tetanus vaccination, other vaccines during pregnancy can protect infants against preventable infections (7). As pertussis antibodies are efficiently transferred from the mother across the placenta to her fetus (6), vaccination of pregnant mothers with a single dose of toxoid, diphtheria toxoid, and acellular pertussis (Tdap) between 28 and 38 weeks of gestation, is one of the strategies to control and protect newborn infants against pertussis (3).
Complications related to neonatal pertussis are atypical yet life threatening, including prolonged hospitalization, pneumonia, seizures, encephalopathy, and death in very young infants (1). Moreover, lower concentrations of pertussis antibodies and insufficient antibodies transport to neonates may increase the risk of pertussis in infants aged < 2 months.
2. Objectives
As in Iran injection of Tdap vaccine for the adult population is not routine (8), the primary objective of the current study was to determine the status of maternal-neonatal immunization by estimation of specific pertussis antibodies in mothers and their infants.
3. Methods
A cross-sectional descriptive analytical study was carried out in the delivery room of Vali e Asr hospital as a tertiary referral center, and also a teaching hospital affiliated to Tehran University of Medical Sciences (Tehran-Iran) from October 2015 to February 2016. The target population included term-healthy neonates with their mothers. Inclusion criteria were maternal age of between 18 and 45 years and gestational age of 37 to 42 weeks. All subjects gave informed consent before entering the study. The exclusion criteria included twin pregnancy, preterm delivery, systemic and chronic diseases, or congenital anomalies in the neonates. Neonatal physical examination and anthropometric assessments were done by an expert neonatologist. Demographic characteristics of participants, such as neonates’ birth weight, height, gestational age, mode of delivery, mother’s age, parity, and history of childhood or antenatal vaccination were recorded in checklists. Immediately after birth, 5 mL of mother’s blood and 2 mL of umbilical cord blood were drawn, labeled, and transferred to the laboratory of pediatric infectious diseases research center to assay the mother and neonate’s serum pertussis antibodies. Samples were centrifuged and stored at -80°C. Mothers and neonates’ serum pertussis toxin (PT) IgG and filamentous hemagglutinin (FHA) IgG were assessed by Bordetella pertussis IgM, IgGenzyme-linked immune sorbent assay Kit (manufactured by IBL international; Germany, sensitivity; 1 u/mL).
To determine recent infection, mothers’ IgA-PT and IgA-FHA were also measured. The results were classified as negative, positive, and equivocal based on the kit cut-off values (9). For further analysis, measured values were log-transformed and results were reported as geometric mean concentrations (GMC). Finally, the correlations between the mean concentrations of pertussis antibodies in paired mother and cord blood samples were assessed and compared, as well as their immunization status.
The SPSS software package version 19 was used to perform the statistical analysis. The Chi-Square test (for comparing 2 categorical variables, such as frequency of seropositives between mothers and their children) and logistic regression were applied where applicable; the relationship of continuous variables, such as mother’s age, gestational age, and anti-body titer were analyzed using the Pearson correlation test. To compare level of antibody titers between the mother and her child, paired t test was performed. Data are presented as mean ± standard deviation for continuous variables and n% for categorical variables. Antibody titer was changed to a logarithm and mean geometric-standard deviation was calculated and detected. Based on other studies (10, 11) and the mentioned formula by Edwin Bidwell Wilson (1927), with the proposed sample size of 88, the study had a power of 90%.

The level of significance was considered at P < 0.05. The study was approved as a student thesis (ID; IR.SBMU.RAM.1394.556) by the Medical ethics committee of Shahid Beheshti University of Medical Sciences, according to the Helsinki declaration. The gathered data were confidential and no extra burden was constrained on the participants.
4. Results
Eighty-eight term parturient mothers (mean age of 29.42 ± 5.12 years) with their newborns were entered in the study. Mean gestational age was 38.16 ± 2.55 weeks and type of delivery in 69 subjects (78.41%) was cesarean section. Neonates’ mean birth weight was 3244 ± 571 grams and 41 cases (46.59%) were male. Seventy-eight mothers (88.64%) had childhood vaccination (under 6 years old) while others could not remember any childhood vaccination. Of all subjects, 48 mothers (54.5%) were also vaccinated during pregnancy (diphtheria- tetanus). None of the participants reported a history of pertussis or pertussis in their families; however, 8 subjects had prolonged cough and 4 cases had other respiratory complications, including dyspnea or asthma. According to the cut off, PT IgG in 33%, 13.6%, and 53.4% of mothers were negative, equivocal, and positive, respectively. Tables 1and 2 demonstrate the mean concentration and status of pertussis antibodies in mothers and umbilical cord blood. No correlations were seen between the level of antibody and maternal age (P = 0.158), parity (P = 0.182), gestational age (P = 0.238) or neonate’s birth weight (P = 0.064).
| Participants (n = 88) | FHA IgG (GMC) | PT IgG (GMC) |
|---|---|---|
| Mothers (n = 88) | 15.96 | 41.07 |
| Newborns (n = 88) | 29.09 | 13.23 |
| P value | 0.000 | 0.000 |
Abbreviation: GMC, Geometric Mean Concentration.
| Participants, N = 88 | PT IgG | FHA IgG | |||
|---|---|---|---|---|---|
| Neg < 16 | EQ 16 - 24 | Pos > 24 | Neg. < 25 | Pos > 25 | |
| Newborns, n = 88 | 54 (61.4) | 13 (14.8) | 21 (23.9) | 60 (68.2) | 28 (31.8) |
| Mothers, n = 88 | 29 (33) | 12 (13.6) | 47 (53.4) | 57 (64.8) | 31 (35.2) |
| PT IgA | FHA IgA | ||||
| Neg < 8 | EQ 8-12 | Pos > 12 | Neg < 42 | Pos > 42 | |
| 80 (90.9) | 4 (4.5) | 4 (4.5) | 84 (95.5) | 4 (4.5) | |
Abbreviations: Neg, Negative; EQ, Equivocal; Pos, Positive
aValues are expressed as No. (%).
Results showed that the incidence of pertussis in the current study was 4.5%; of all mothers, with regards to both PT IgA and FHA IgA, 4 cases had recent pertussis and more than 90% of them did not show antibodies related to acute pertussis infection (Table 2).
Significant positive correlations were observed between mother and neonate’s FHA IgG and PT IgG (P value < 0.001; r = 0.66 and P value < 0.001; r = 0.53, respectively); higher cord blood FHA IgG and PT IgG were observed among neonates born from mothers with higher FHA IgG and PT IgG. This correlation with regards to PT IgG of maternal and cord blood was also significant (P value < 0.001 and r = 0.53). Detailed data are shown in Table 1.
Tables 3 and 4 demonstrate detailed data related to correlations between maternal blood and cord blood PT IgG and FHA IgG; seropositive antibody were observed more frequently among neonates with PT seropositive mothers.
| Mothers’ PT IgG | Neonates’ PT IgG | P Value | ||
|---|---|---|---|---|
| Neg | EQ | Pos | ||
| Negative (n = 29) | 24 (82.76%) | 3 (10.34) | 2 (6.89) | <0.001 |
| Equivocal (n = 12) | 8 (66.67) | 1 (8.33) | 3 (25) | |
| Positive (n = 47) | 22 (46.81) | 9 (19.48) | 16 (34.04) | |
Abbreviations: Neg, Negative; EQ, Equivocal; Pos, Positive
aValues are expressed as No. (%).
| Mothers’ FHAIgG | Neonates’ FHA IgG | P Value | |
|---|---|---|---|
| Neg | Pos | ||
| Negative, n = 57 | 51 (89.47) | 6 (10.53) | 0.061 |
| Positive, n = 31 | 9 (29.03) | 22 (70.97) | |
Abbreviations: Neg, Negative; Pos, Positive
aValues are expressed as No. (%).
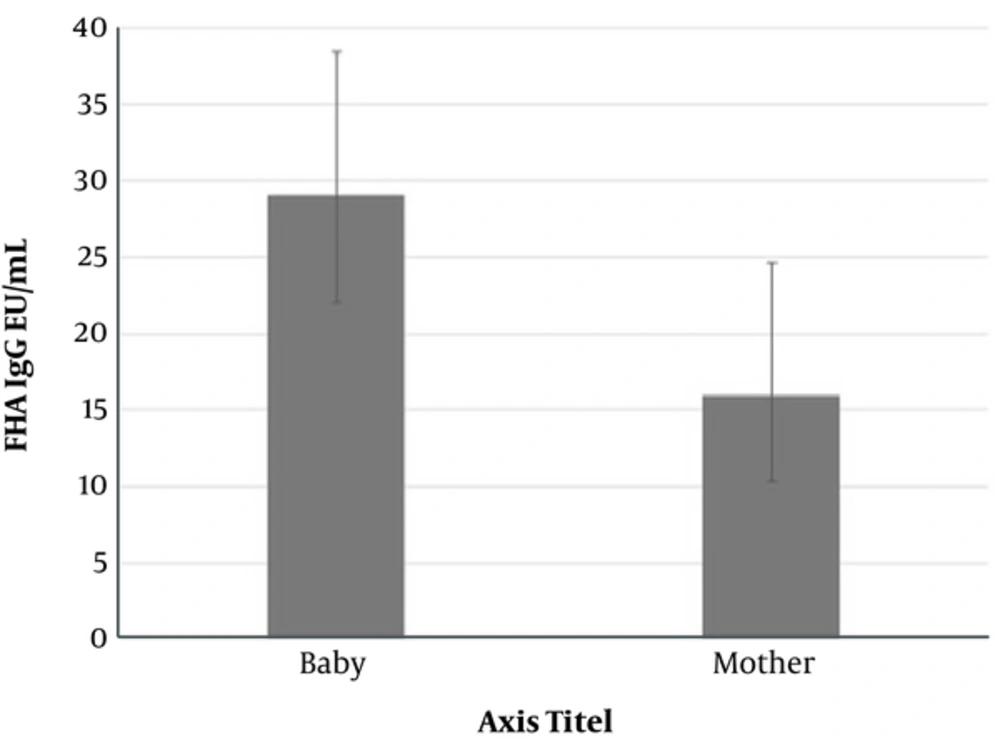
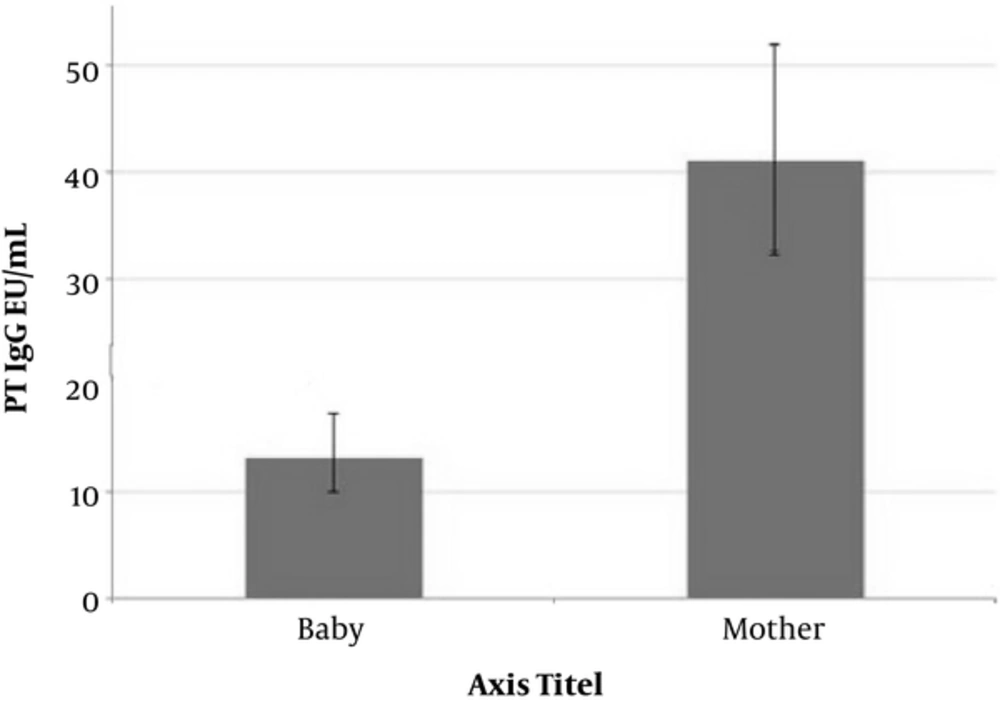
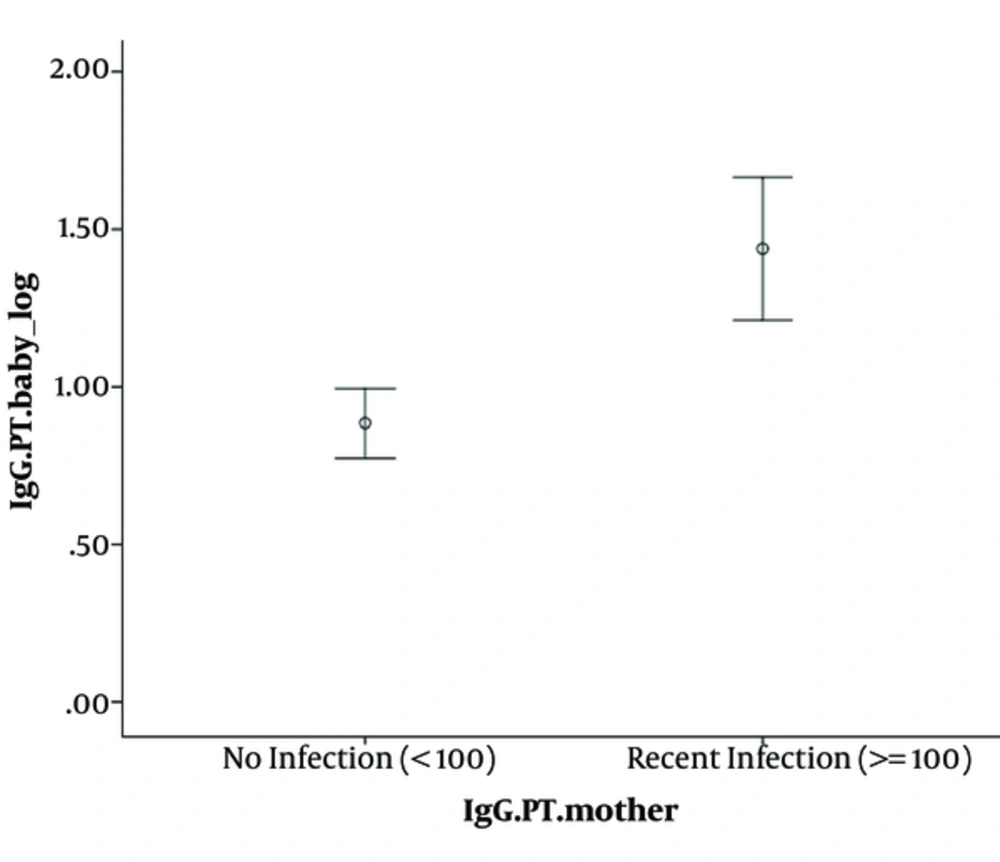
Maternal and cord blood geometric mean titers for FHA IgG and PT IgG are shown in Figures 1 to 3; the geometric mean titer for FHA IgG in cord blood was 1.72 times higher than in the mother’s blood. This increase was statistically significant (P value < 0.001). On the other hand, geometric mean titer for PT IgG in maternal blood was 3 folds higher than in cord blood (P value < 0.001). As shown in Figure 3, geometric mean titers for PT IgG was considerably higher among neonates, whose mothers had PT IgG > 100 Eu/mL than in neonates, whose mothers had PT IgG < 100 Eu/mL. This result showed a positive correlation between paired mother and cord blood (P value < 0.001).
5. Discussion
Many countries, including Iran, with high vaccine coverage have been exposed to Bordetella pertussis resurgence. With decreased immunity after 10 years of vaccination, age of incidence shifts from childhood to adulthood. Moreover, lack of immunity in females during their reproductive age leads to insufficient passive immunity in their infants (12). On the other hand, in other countries such as Canada, Switzerland, USA, United Kingdom, and Australia, acellular pertussis (aP) vaccine was recommended and scheduled for adults as well as pregnant females as a booster dose (13). Many investigations pointed to different strategies for the control of pertussis transmission. Eberhardt et al. showed an efficient neonatal seropositivity, following second versus third-trimester immunization (6). Immunization of pregnant females with Tetanus Diphtheria and Acellular Pertussis (Tdap) in the third trimester was also proposed as tolerable and appropriate immune responses (5). The immunization of newborns at birth is another possible strategy (13).
Based on the results, 81% of mothers were vaccinated during their childhood, however, both FHA-IgA and PT-IgA (indicating a current exposure) were positive in only 4.5% of subjects. Other studies from Iran also demonstrated such a low level of immunity among pregnant females. Hashemi et al. showed that among 288 pregnant females, only 103 (35.8%) were seropositive (antibody > 24 U/mL) (10). Van de Wielen also showed that natural or vaccine-induced immunity against pertussis is not protective for a long time and adults are susceptible to infection (14).
Regarding the cord blood PT-IgG, 61.4% of newborns in the present study were unprotected against B. pertussis. This rate is comparable with other studies. Plans et al. showed that 90% of newborns were unprotected according to anti-PT cord blood samples (11). Shakib et al. demonstrated that in spite of effective maternal antibody transfer, 75% of 81 newborns had lower level of pertussis antibody than that required for protection. This rate was increased to 90% at age of 6 weeks (15). Smallenburg et al. showed a decreasing trend in PT-IgG concentration after birth. This measure was 60.1 U/mL, 40.6 U/mL, 20.7 U/mL, and 16.7 U/mL in umbilical cord blood, at the age of 5 days, 1 month, and 2 months, respectively (16).
As shown in Figure 3, geometric mean titer for PT-IgG was considerably higher among neonates, whose mothers had PT-IgG of > 100 Eu/mL compared to neonates, whose mothers had PT IgG < 100.
This result showed a positive correlation between paired mother and cord blood. This significant relationship between maternal anti-PT with neonatal cord blood was confirmed by Gonik et al. (17). Moreover, it has been shown that in spite of the highly efficient placental antibodies transportation, IgG concentrations are not high enough to passively protect neonates in the first months of life (1).
This study found that GMC of IgG to FHA in the cord serum was significantly higher than in the mother’s blood. The geometric mean titer for FHA IgG in the cord blood was 1.72 times more than that of the mother’s blood, which may indicate active transport of antibody across the placenta. Other studies confirmed the current finding (1, 17, 18). Gonik et al. indicated higher geometric mean titers for FHA IgG in cord blood than in maternal blood (32 vs. 26.6). Healy et al. also showed more than 178% active placental transport of FHA IgG from the mother to the fetus at the term of gestation (P < 0.001) (1).
This research found that the geometric mean titers for PT IgG in maternal blood were 3 folds higher than in cord blood. Consistent with the current results, Mooi et al. indicated that maternal transfer of PT IgG antibodies was lower than that required to protect neonates against pertussis (19). On the other hand, some studies showed different results; Kurugol et al. showed higher GMCs of IgG (1.3 times) in cord blood than in parturient mother’s blood (20). Villarreal-Pérez et al. demonstrated a higher concentration of IgG anti-PT in the umbilical cord than in the mother’s blood (4.3% versus 1.4%) (21). Fallo et al. also revealed a ratio equal to 1.18 for the cord blood IgG-PT GMC compared with the maternal level (22).
The limitation of the current study was that it did not follow the neonates to determine age-specific profile after birth. Levels of antibodies to PRN in serum were not measured and the authors also did not consider ethnicity; however, other studies noted the role of pertussis-specific IgG on pertussis incidence (23). Such information could provide more beneficial data.
Results of the present study demonstrated a low level of immunity against B. pertussis in the studied population; 95.5% of mothers and 61.49% of their neonates were unprotected. Such a low level of immunity may increase the risk of pertussis morbidity in very young infants. Implementing some strategies during the antenatal period seem to be of great importance and further investigations regarding safety of feto-maternal immunization against B. pertussis are strongly proposed.