1. Background
Since December 2019, with the outbreak of the COVID-19 pandemic all over the world, there have been many challenges in the treatment of this fatal viral infection (1). The most common symptoms of infants with COVID-19 infection were fever, cough, retinitis, weakness, and gastrointestinal symptoms (2). Regarding the new formation of this generation of coronavirus, several treatment options have been suggested to prevent the mortality and severe morbidities of this virus. Remdesivir is a broad-spectrum antiviral agent initially synthesized and developed by Gilead Sciences as a treatment for Ebola virus infection in 2014 (3). Its antiviral mechanism is mainly the result of the delayed chain cessation of the nascent viral RNAs and then the inhibition of the virus (4). Remdesivir is a promising treatment option in both adults and children infected by this virus, except for neonates and early infants, which has not been determined yet. The reported complications of Remdesivir consisted of skin rashes (< 2%), nausea (3 - 7%), prolonged prothrombin time (9%), increased serum alanine aminotransferase (3%), increased serum aspartate aminotransferase (6%) (5), hypersensitivity reaction (< 2%), seizure (< 2%), anaphylaxis, angioedema, and bradycardia (including severe bradycardia and sinus bradycardia) (6), cardiac failure, and hypotension (7). The contraindications of drug administration are hypersensitivity to Remdesivir or any components of the formulation.
According to the existing recommendations, the administration of this drug was limited in the first months of life in neonates and early infants. Regarding the concerns for the fatal complications of the disease, and due to the lack of known effective drug treatments, in some situations, the use of Remdesivir is obligatory.
2. Objectives
In this case series, we present a cohort of 15 neonates with COVID-19 who were admitted to our hospital. These neonates received treatment with Remdesivir, and we aimed to evaluate the effectiveness and potential side effects of this drug in our population.
3. Methods
This case series study was conducted at Mofid Children's Hospital, affiliated with Shahid Beheshti University of Medical Sciences, from April 2021 to January 2022. Our neonatal intensive care unit is a referral center for patients with medical or surgical problems. Our ward has a specific line for care provision for neonates with COVID-19. Inclusion criteria were neonates with a history of administration of Remdesivir due to COVID-19, neonates with severe respiratory distress needing mechanical ventilation, and neonates with a prolonged need for supplemental oxygen and hospitalization. In patients with a short duration of fever, poor feeding, or other mild symptoms of neonatal sepsis due to COVID-19, just supportive therapy was done. These patients were excluded from the study. The decision for the administration of Remdesivir must be confirmed by consulting a pediatric infectious disease specialist and the clinical pharmacist of our hospital, besides the neonatologist's order. Written consents were obtained from the parents. Before and after the drug administration, the patients underwent complete blood cell count (CBC), blood urea nitrogen (BUN), creatinine, and liver function tests. In some patients, other complementary laboratory tests, such as lactate dehydrogenase (LDH), ferritin, Di-dimer, total protein, and albumin, were performed as well. We prescribed the drug with a 2- to 3-h infusion of a loading dose of 5 mg/kg on the first day, followed by 2.5 mg/kg/day for 4 days, concomitant with continuous cardiac monitoring and blood pressure monitoring every 15 min. In the most severe form of the disease, not responding to the 5-day course of treatment, a 10-day treatment by Remdesivir, or other treatment options such as corticosteroid, ACTEMRA, and surfactant were considered.
4. Results
In the current research, we present 15 neonates admitted to our hospital with a history of administration of Remdesivir due to COVID-19 with severe respiratory distress. The mean gestational age of the patients was 37.8 weeks, their mean birth weight was 2884 g, and 7 of them were female. Table 1 shows the demographic and clinical characteristics of the patients. As we have shown, all of the cases have a birth weight of > 2000 g at the time of drug administration.
| Patients No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational age, w+d | 37+5 | 38 | 39 | 37 | 34 + 2 | 38 | 39 + 5 | 39 | 40 | 39 | 38 | 36 | 38 | 37 + 6 | 38 |
| Sex | M | M | F | F | F | M | M | F | M | M | F | M | F | M | F |
| Birth weight, g | 2880 | 3400 | 2800 | 2900 | 2270 | 3170 | 3560 | 2800 | 2000 | 3300 | 3800 | 1800 | 3000 | 2780 | 2800 |
| Age of treatment, d | 10 | 98 | 23 | 12 | 25 | 5 | 32 | 8 | 38 | 20 | 31 | 19 | 17 | 4 | 4 |
| Clinical Manifestation | |||||||||||||||
| Cyanosis | * | - | - | - | - | - | - | - | - | - | - | - | * | - | - |
| Respiratory distress | - | * | * | * | * | * | * | * | * | - | * | * | * | * | - |
| Fever | - | - | - | * | - | - | * | * | - | * | - | - | * | * | - |
| Diarrhea | - | * | - | * | - | - | - | - | - | - | - | - | - | - | - |
| Lethargy | - | - | - | - | - | - | - | * | - | - | - | - | - | - | - |
| Cyanosis | - | - | - | - | - | - | - | - | - | * | - | - | - | - | - |
| Cough | - | - | - | - | - | - | - | - | - | * | - | - | * | * | * |
| Dehydration | - | * | - | - | - | - | - | - | - | - | * | - | - | - | - |
| Seizure | - | - | - | - | - | - | - | - | - | - | * | - | - | - | - |
| Poor feeding | - | * | - | - | - | - | - | - | - | - | - | - | - | * | - |
| Specific Radiological Findings | |||||||||||||||
| CT scan and X-ray | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Laboratory Findings Before and After Treatment | |||||||||||||||
| WBC | |||||||||||||||
| Before | 8000 | 800 | 11500 | 13300 | 3100 | 6800 | 15800 | 10600 | 7000 | 7400 | 8500 | 14200 | 11700 | 5400 | 6600 |
| After | 11500 | 9600 | 920 | 10200 | 4500 | 9300 | 9300 | 11300 | 11300 | 13600 | 11400 | 12100 | 17000 | - | - |
| PMN% | |||||||||||||||
| Before | 45 | 70.5 | 0 | 51 | 20 | 70 | 66 | 60 | 82 | 40 | 58 | 75 | 47 | 30 | - |
| After | 35 | 78 | 0 | 40 | 23 | 36 | 40 | 66 | 44 | 44.1 | 72 | 52 | 50 | - | - |
| LYM | |||||||||||||||
| Before | 40 | 19.7 | 39 | 45 | 74 | 21 | 24 | 31 | 10 | 13.2 | 35 | 17 | 49 | 62 | - |
| After | 58 | 21 | 55 | 30 | 69 | 53 | 50 | 25 | 42 | 10 | 35 | 38.9 | 42 | - | - |
| Hgb | |||||||||||||||
| Before | 14.1 | 15.4 | 11.3 | 14 | 8.9 | 14.9 | 10.4 | 12.5 | 11.3 | 13.2 | 12.2 | 15.4 | 13.4 | 14.5 | 12.1 |
| After | 11.7 | 12.2 | 9.9 | 10.7 | 10.4 | 12.4 | 12.7 | 12 | 9.5 | 10 | 7.9 | 11.5 | 14.4 | - | - |
| HCT | |||||||||||||||
| Before | 41.2 | 45.4 | 35.3 | 39.2 | 24 | 40.9 | 32 | 37.9 | 34.1 | 39.5 | 35.9 | 44.1 | - | 42.8 | 34.1 |
| After | 33.1 | 38.6 | 32.4 | 31.4 | 30 | 34.6 | 39.6 | 35 | 31 | 32.2 | 24.6 | 44.1 | - | - | - |
| PLT | |||||||||||||||
| Before | 292000 | 34000 | 366000 | 366000 | 73000 | 200000 | 59000 | 243000 | 487000 | 539000 | 277000 | 35.800 | 434000 | 459000 | 436000 |
| After | 562000 | 37000 | 391000 | 538000 | 123000 | 628000 | 150000 | 380000 | 510000 | 526000 | 8900 | 362000 | - | - | - |
| CRP | |||||||||||||||
| Before | 14 | 50 | 9 | 13 | 42 | - | 45 | 11 | 7 | 1.1 | 1 | 2 | 37 | 31 | 1 |
| After | 8 | 32 | 25 | 9 | 39 | - | 18 | 8 | 21 | 3 | - | 5 | 3 | 3 | |
| BUN | |||||||||||||||
| Before | 19.3 | 8.8 | 5 | 4 | 8.9 | 8.2 | 12 | 13.3 | 19.1 | 10.9 | 14 | 10 | 4 | 2.1 | 6.2 |
| After | - | 21.3 | 7.5 | 1.3 | 5 | 4.4 | 11.3 | 6.7 | 22.2 | - | 31.2 | 5.6 | 3 | 3 | - |
| CR | |||||||||||||||
| Before | 0.6 | 0.3 | 0.5 | 0.4 | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.7 | 0.4 | 0.8 | 0.3 | 0.51 | 0.34 |
| After | - | 0.4 | 0.5 | 0.3 | 0.3 | 0.5 | 0.4 | 0.5 | 0.42 | - | 2.1 | 0.5 | 0.5 | 0.48 | - |
| SGOT | |||||||||||||||
| Before | - | - | 63 | - | - | 28 | 30 | - | 27 | - | - | 117 | 39 | - | - |
| After | 60 | 81 | 38 | 31 | - | - | 42 | 51 | 87 | 68 | 254 | - | 34 | 37 | - |
| SGPT | |||||||||||||||
| Before | - | - | 35 | - | - | 12 | 16 | - | 57 | - | - | 57 | 19 | - | - |
| After | 28 | 186 | 27 | 24 | - | - | 24 | 14 | 86 | 50 | 42 | - | 24 | 23 | - |
| ALK | |||||||||||||||
| Before | - | - | 286 | - | 707 | - | - | - | - | - | - | 745 | 218 | - | - |
| After | 400 | 426 | 339 | 200 | - | - | 594 | - | 394 | 539 | 451 | - | 302 | 458 | - |
| Ferritin | |||||||||||||||
| Before | - | - | - | - | - | 287 | 800 | - | - | - | - | - | - | - | - |
| After | 200 | 800 | 800 | 800 | 800 | - | - | 800 | - | - | - | - | - | - | - |
| d-dimer | |||||||||||||||
| Before | - | - | - | - | - | - | 200 | - | - | 422 | - | - | - | - | - |
| After | - | - | - | - | - | - | - | - | 200 | - | - | - | - | - | - |
| CPK | |||||||||||||||
| Before | - | - | - | - | - | - | - | - | - | 1660 | - | - | - | - | - |
| After | 0.96 | - | - | - | 25 | - | - | 98 | - | - | 1168 | - | - | 77 | - |
| LDH | |||||||||||||||
| Before | - | - | - | - | - | 937 | 2299 | - | 1017 | - | - | - | 626 | - | - |
| After | 605 | - | - | 2021 | 981 | - | - | 2587 | - | - | 3050 | - | 868 | 759 | - |
| Troponin | |||||||||||||||
| Before | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| After | 0.114 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fibrinogen | |||||||||||||||
| Before | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| After | 269 | - | - | - | - | - | - | - | - | 177 | - | - | - | - | - |
| Alb | |||||||||||||||
| Before | - | - | 3.9 | - | - | - | - | - | - | - | - | - | - | - | - |
| After | - | - | 3.3 | - | 2.4 | - | 3.6 | - | 3.4 | - | - | - | - | - | - |
| Total Pr | |||||||||||||||
| Before | - | - | 6.2 | - | - | - | - | - | - | - | - | - | - | - | - |
| After | 4.6 | - | 5.4 | - | 4 | - | - | - | - | - | - | - | - | - | - |
| Positive PCR COVID-19 | |||||||||||||||
| Before | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| After | No | No | No | No | Yes (4 times) | No | Yes (6 times) | No | No | No | Yes | Yes | No | No | Yes |
| Duration of treatment, d | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 10 | 14 | 5 | 5 | 5 | 6 | 5 | 5 |
| Survival | Alive | Alive | Death | Alive | Alive | Alive | Death | Alive | Death | Alive | Death | Death | Alive | Alive | Alive |
| Interval between death and start of Remdesivir, d | - | - | 80 | - | - | - | 45 | - | 54 | - | 1 | 33 | - | ||
| Other Treatment of COVID-19 | |||||||||||||||
| Dexamethasone | - | * | - | * | * | * | * | * | * | - | * | * | * | * | * |
| IVIG, G | - | - | - | - | * | - | - | - | * | - | - | - | - | ||
| Surfactant | - | - | - | - | - | - | - | * | - | - | - | - | - | ||
| Actemra | - | - | - | - | - | - | - | * | - | - | - | - | - | ||
| Prednisolone | - | - | - | - | - | - | - | - | - | * | - | - | - | ||
| Drug’s side effects | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Underlying medical condition | No | No | Yes | No | No | No | Yes | No | Yes | No | Yes | Yes | No | No | No |
Abbreviations: CT, computed tomography; WBC, white blood cell; PMN, polymorphonuclear; LYM, lymphocyte; Hgb, hemoglobin; HCT, hematocrit; PLT, platelet; CRP, C reactive protein; BUN, blood urea nitrogen; CR, creatinine; SGOT, serum glutamic-oxaloacetic transaminase; ALK, alkaline phosphatase; CPK, creatinine phosphokinase; LDH, lactate dehydrogenase; Alb, Albumin; Pr, protein; PCR, polymerase chain reaction; IVIG, intravenous immunoglobulin.
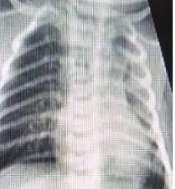
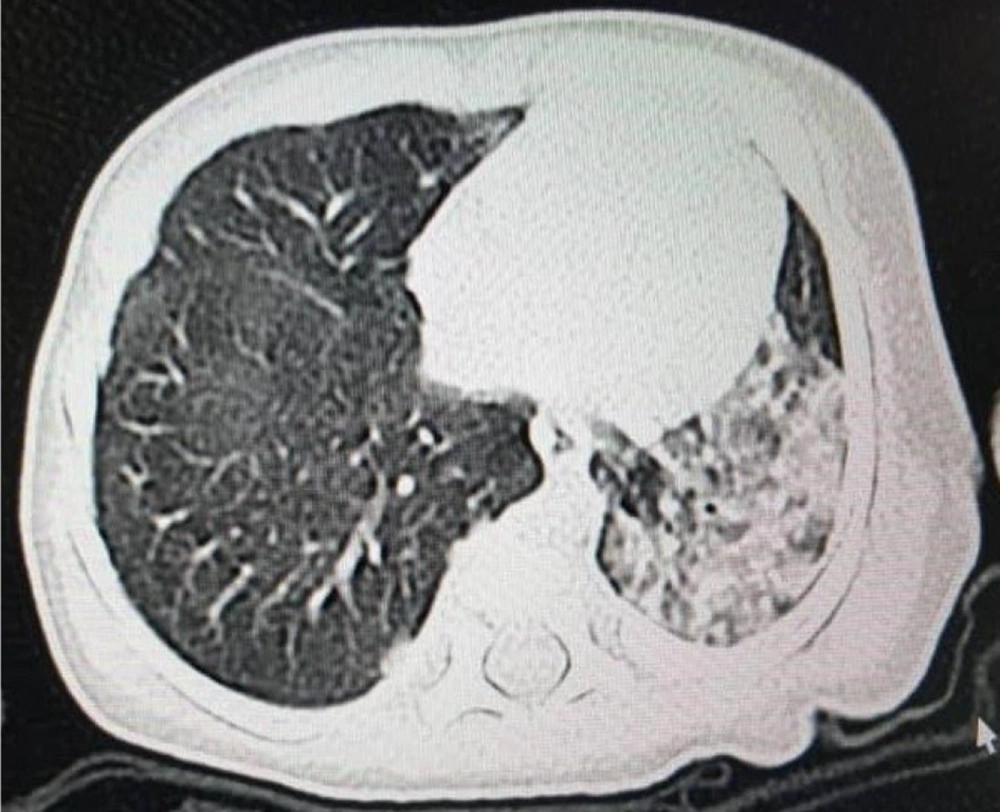
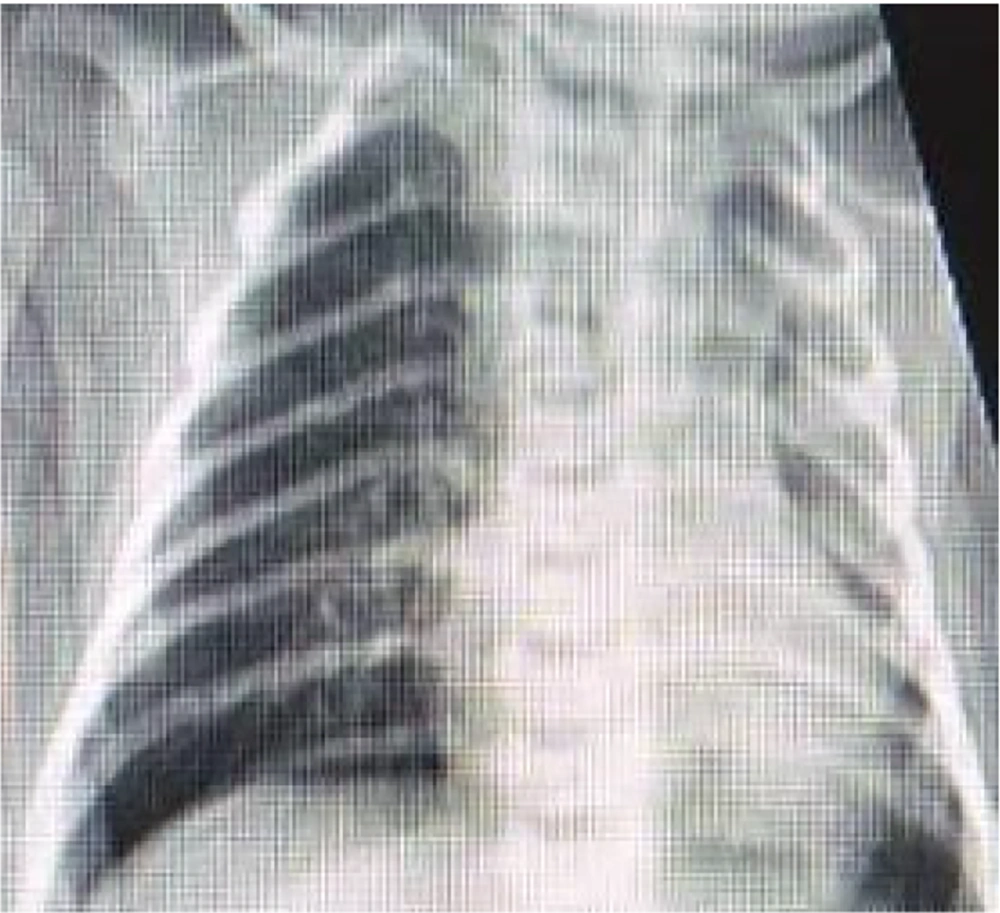
The mean post-natal age of the patients when we started Remdesivir was 23.3 days (from 5 to 98 days). All patients included in the study exhibited respiratory distress and cyanosis and required oxygen supplementation or mechanical ventilation. Additionally, positive radiologic findings in chest X-ray or chest computed tomography (CT) scan supported the diagnosis of COVID-19 in these patients. Of the total patients, 12 cases with respiratory distress and 6 patients with fever (temperature > 37.5°C) were detected. With the exception of 3 patients, all individuals included in the study had a positive polymerase chain reaction (PCR) test for COVID-19 prior to receiving the drug. For cases 10, 13, and 14, even though the PCR test results were negative, the administration of Remdesivir was still prescribed. This decision was made due to either a positive maternal history of COVID-19 or the severe involvement of the neonate in question. Figures 1 and 2 show the severe pulmonary involvement of this patient in the lung CT scan and chest X-ray. The duration of drug administration was 4 to 6 days in 8 patients. Due to the severe course of the disease, 2 patients received drugs for 10 and 14 days.
For 12 patients, besides Remdesivir, dexamethasone was ordered as well. For 1 patient, due to severe respiratory distress and respiratory failure resistant to Remdesivir, 2 courses of endotracheal surfactant (Beraksurf) were instilled, and due to the persistent respiratory distress, 1 dose of Actemra was prescribed besides the other supportive care, such as invasive and non-invasive mechanical ventilation. In conclusion, the aforementioned treatments resulted in successful recovery. Following the administration of the drug, PCR test results for COVID-19 were negative in 10 patients, indicating successful clearance of the virus. However, in 5 cases, the PCR test continued to yield positive results, suggesting the persistent presence of the virus despite treatment.
We observed a 3-fold increase in the BUN value for case 2 and a 2-fold increase for case 11. Additionally, in case 11, there was a more than 5-fold increase in the creatinine value. Case 2 was a term male infant who was hospitalized with respiratory distress, poor feeding, severe diarrhea, and dehydration. Also, case 11 was a term female infant who was hospitalized with underlying heart disease and severe respiratory distress, dehydration, and seizure. Therefore, both of them were so ill.
Regarding the recovery of all the patients treated with Remdesivir, it seems that the effectiveness of this drug is confirmed, although the effect of other concomitant treatments should not be overlooked. It should be noted that after long periods of hospitalization, 5 patients died due to the underlying congenital malformation (case 3, pulmonary airway malformation; case 7, congenital gastrointestinal and heart anomaly; cases 9 and 11, congenital heart anomalies; case 12, hydrocephaly and intrauterine growth restriction [IUGR]). The deaths of these patients have not been related to COVID-19 disease or its treatment. Fortunately, there were no reported side effects, such as skin rashes, nausea/vomiting, the impairment of liver or kidney function, hypersensitivity reaction (anaphylaxis and angioedema), seizure, bradycardia, cardiac failure, hypotension, or hypertension.
None of our patients needed dosing adjustment due to renal or liver function impairments. In the long-term follow-up of the surviving patient (10 months or more), there have been no detected side effects of this drug so far.
5. Discussion
In this research, we present 15 neonates with COVID-19 who were admitted to our hospital. These neonates required administration of Remdesivir due to severe respiratory distress, which necessitated either invasive or noninvasive mechanical ventilation, as well as supplemental oxygen, within the first days of life. The efficacy of Remdesivir in the treatment of COVID-19 in adults has been confirmed (8), but the safety and effectiveness of this drug in children and neonates have not been approved. Despite this, in the most recent guidelines for the drug treatment of COVID-19, Remdesivir is recommended for infants with birth weights over 3500 g or over 2 - 3 months old. In the recent COVID-19 pandemic, we had to administer this drug to our patients as a lifesaving modality. Fortunately, during the hospital stay and with a 10-month interval after the administration of this drug, there were not any unwanted complications, such as cardiac, gastrointestinal, hepatic, renal, dermal, central nervous system disturbance, hypersensitivity, etc. It seems that the usage of this drug during the first months of life is safe. Sinus bradycardia is one of the reported side effects of Remdesivir in adults (9). Fortunately, we did not encounter any cases of significant bradycardia in our patients.
Recent research regarding the treatment of COVID-19 suggests that the drug be prescribed in the first 7 days of the disease, as early as possible (10). Our patients received the drug during the first 7 days of illness, while the mean interval of the drug initiation was 2 days, although, for 12 patients besides Remdesivir, other drugs such as corticosteroid, surfactant(for 1 patient) and Actemra (for 1 patient) were used, too.
The mean hospital stay of our patients after the administration of Remdesivir was 23.7 days, ranging from 7 to 80 days. This time interval is a good opportunity for monitoring the probable side effects of the drug.
Although 5 patients died during hospitalization, their underlying diseases (congenital airway, gastrointestinal, and cardiac malformations) were the causes of their deaths; their deaths were not related to COVID-19 or its treatment.
Méndez-Echevarría et al. have conducted a cohort study in Spain as titled: "The Compassionate Use of Remdesivir in the Children with COVID-19" in 2021, a multicenter observational study on the children with confirmed SARS-CoV-19. They compared the drug effectiveness in 3 infants and 1 child admitted to the hospital. They reported no drug-related adverse effects in their patients. In their study, 1 patient with serious clinical status died. They suggested an urgent clinical trial of Remdesivir therapy for children. In this study, the minimum age of the patients was 1.5 months (11).
In a case report by Saikia et al., 2 ex-premature infants with gestational ages of 31 and 33 weeks and body weights of 2700 and 1500 g, respectively, were described. These infants developed pulmonary disease as a result of COVID-19 during the second and fifth weeks of life. They were treated with Remdesivir with no complications. The liver and kidney functions, as well as the complete blood count (CBC), of the patients were within the normal range both before and after the treatment (12). Similarly, in the laboratory examinations conducted on the patients in our study, no abnormalities or disturbances were detected in blood, kidney, or liver tests.
In a systematic review and meta-analysis conducted by Prateek Kumar Panda, which included 8243 children with COVID-19, Remdesivir was found to be the most commonly used antiviral drug. It was administered in 6.2% of the patients, and no serious adverse effects were reported. This research showed that among pharmacological modalities, antivirals (such as Remdesivir) and anti-inflammatory agents (such as corticosteroids) have the most promising evidence of severe cases of pediatric COVID-19 (13).
5.1. Limitations and Conclusions
One limitation of our report is the retrospective nature of the study, as we analyzed data from patients who had already received Remdesivir. Additionally, the sample size of our study was small, which may impact the generalizability of the findings. We suggest future clinical trials as the best methodology for the evaluation of the efficacy and safety of this drug. It seems that the use of Remdesivir in the treatment of severe forms of neonatal and early infancy COVID-19 is safe and effective.