1. Context
Multi-system inflammatory syndrome in children (MIS-C) is a seemingly rare but severe condition that has emerged as a significant concern associated with the COVID-19 pandemic (1). This novel clinical syndrome first emerged in April 2020 as a post-COVID-19 multisystem disorder. Since its emergence, the medical community has faced a new, concerning phenomenon (2). Multi-system inflammatory syndrome in children is characterized by widespread inflammation affecting multiple organ systems in children and adolescents (3). The disease arises as a post-infectious immune dysregulation primarily associated with prior SARS-CoV-2 infection in children. The pathophysiological cascade involves an exaggerated immune response, notably the overactivation of T cells and macrophages. These immune cells contribute to a systemic inflammatory state impacting various organs. Studies by Dufort et al. (4) and Consiglio et al. (5) delve into the molecular mechanisms. Dufort's research emphasizes the dysregulation of inflammatory cytokines, especially interleukin-6 (IL-6), while Consiglio's work highlights the viral spike protein's role in eliciting immune responses. These findings underscore the intricate interplay between immune cells and specific viral components in the manifestation of MIS-C.
Due to its rapid and severe clinical manifestation in pediatric populations, MIS-C has garnered significant attention, making prompt recognition and treatment imperative for managing this condition (6). Despite the Centers for Disease Control and Prevention (7) continuously tracking and collecting data, and providing updated guidance on this novel disease as new information emerges, the accurate incidence of MIS-C remains challenging due to a lack of comprehensive SARS-CoV-2 testing data (8). As researchers and healthcare professionals strive to unravel the complexities of this syndrome, a robust and evidence-based understanding is imperative to guide effective management and potential preventive strategies. The precise mechanisms leading to MIS-C remain enigmatic due to the numerous clinical presentations and the spectrum of severity in affected children, and are the subject of ongoing research (9). There are numerous uncertainties surrounding MIS-C that still need definitive answers. Considering the scarcity of available information on MIS-C and the evolving nature of knowledge in this area, a comprehensive and up-to-date review of the existing literature is essential to consolidate our current understanding, identify knowledge gaps, and propose avenues for future research.
2. Objectives
We aim to provide an in-depth review of the latest literature on MIS-C to clarify the clinical spectrum, diagnostic criteria, treatment modalities, and management strategies of this novel syndrome.
3. Methods
3.1. Data Sources
This systematic review was performed based on PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines to enhance the validity and transparency of the study (10). We searched the PubMed and Google Scholar databases on December 20, 2022, with no regional or date of publication restrictions. The search terms used were: “Pediatric multisystem inflammatory disease,” “Multisystem inflammatory syndrome in children,” MISC, and MIS-C. The search was repeated on December 25, 2022, to ensure accuracy. The reference lists of all included studies were also searched to identify additional relevant studies.
3.2. Study Selection
Eligibility for inclusion in the study included cross-sectional and cohort studies focused on COVID-19-related MIS-C. Two reviewers independently assessed research titles to exclude studies that did not meet our inclusion criteria before conducting a full review of abstracts and full texts of selected studies. During full-text reviews, studies with insufficient data and those with sample sizes < 10 patients were excluded.
To evaluate the methodological quality of the included studies, we employed the Newcastle-Ottawa Scale (NOS), a widely accepted tool for assessing the quality of non-randomized studies (11). Two independent reviewers appraised each study based on the NOS criteria, which evaluate the selection of study groups, comparability, and ascertainment of exposure or outcome. Any discrepancies in quality ratings were resolved through discussion until consensus was reached. The NOS scores, ranging from 0 to 9 stars, were recorded for each study and used to explore potential sources of heterogeneity in subgroup analyses. Studies with NOS scores ≥ 7 were considered high quality, while those with scores <5 were deemed low quality and subjected to sensitivity analyses to assess their impact on the overall findings.
3.3. Data Extraction
Two independent authors conducted the data extraction process for each article. The information extracted included bibliographic data (title, author/s, publication year), demographic data (age, gender, geographic area, race/ethnicity, comorbidities), clinical data (manifestations and diagnostic information), laboratory data (inflammatory markers, hematology, and cardiac markers), therapeutic management and outcomes data (immunomodulator agents, antibiotics, antiviral medications, and other treatment regimens, hospitalization, admission to pediatric intensive care, mortality). Due to significant variations in data reporting among the included studies, meta-analysis and statistical calculations were not performed.
4. Results
4.1. Study Characteristics
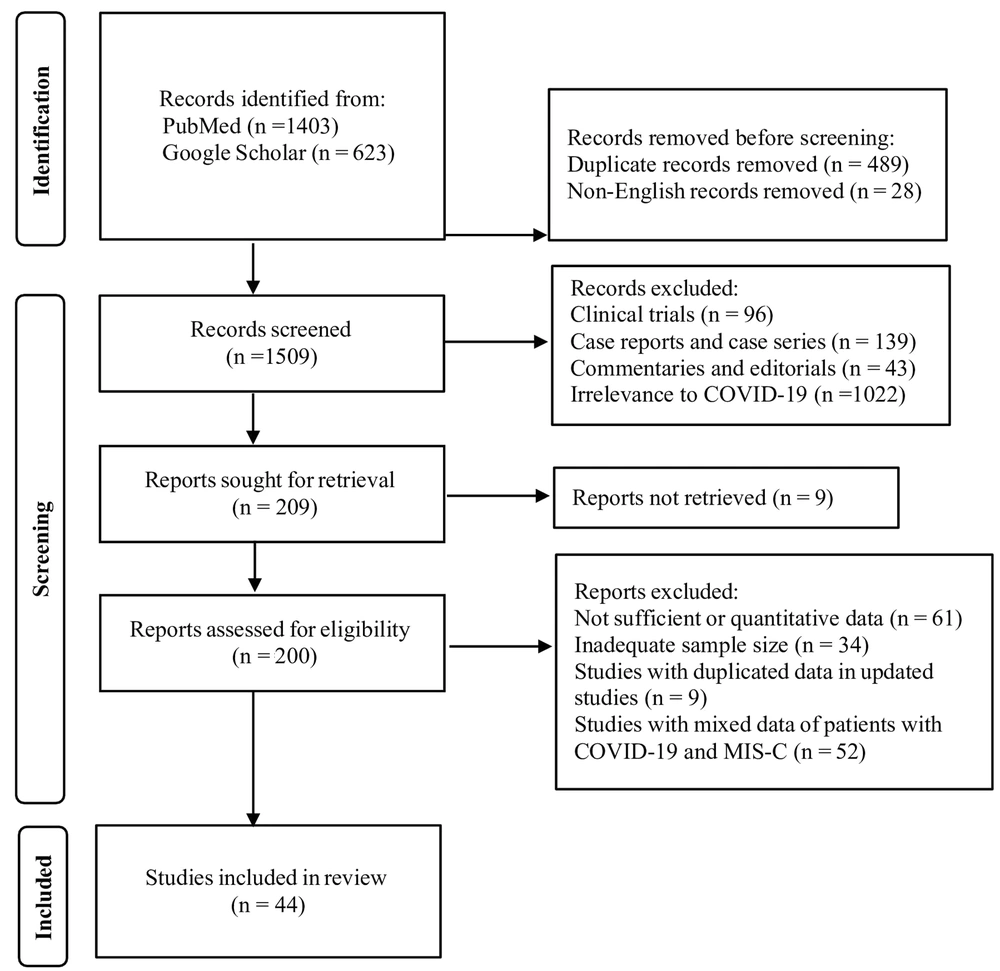
For MIS-C, the search strategy yielded 2026 potentially relevant articles. Four hundred eighty-nine articles were identified as duplicates, and 28 non-English articles were removed. After initial screening of titles and abstracts, 1300 articles were excluded due to study design and irrelevance to COVID-19. Reviewers retrieved and screened the full texts of 200 articles. During full-text screening, 156 articles were further excluded primarily due to absence of quantitative data and inadequate sample size. Additionally, studies with duplicated data from updated sources and those with mixed data of patients with COVID-19 and MIS-C were excluded at this stage.
To meet inclusion criteria, the majority of articles required confirmation of MIS-C that aligned with criteria set forth by the CDC (7). Ultimately, 44 studies (4, 12-54) met inclusion criteria for data extraction (Figure 1). These included 30 retrospective, seven prospective, four cohort, and three cross-sectional studies; all were observational.
Using the NOS to assess study quality, all 44 studies included in this systematic review achieved high-quality ratings, indicating robust methodological rigor in their design and conduct.
4.2. Epidemiological and Clinical Characteristics of Multi-System Inflammatory Syndrome in Children Patients
The analyzed articles provided data on 7297 patients with MIS-C associated with SARS-CoV-2 infection. The included studies collected data from the USA, Turkey, the UK, India, Brazil, Spain, Saudi Arabia, France, Colombia, Israel, Chile, Argentina, and Iran. The United States conducted the most significant number of studies on MIS-C, accounting for 33.3% of the cases. The age range of the children varied from 0 to 21 years, with a median age of 8.6 years. Among the patients in 19 studies, infants aged 0 - 10 months were included. All studies described the gender distribution among the affected children, revealing a total of 4329 male cases (59.2%). Regarding race and/or ethnicity, information on the incidence of MIS-C patients was reported in only 24 (53.3%) of the studies. Among the reported cases, there were 1413 (31.85%) individuals of Hispanic ethnicity and 2127 (50.82%) individuals of non-Hispanic ethnicity. Additionally, there were 1498 (26.84%) individuals of White ethnicity, 1548 (27.74%) individuals of Black ethnicity, 268 (4.8%) individuals of Asian ethnicity, and 180 (3.22%) individuals belonging to other ethnicities (Table 1).
| Study No | First Author (Publication Year) | Number of Patients/Males | Age (y) | Race/Ethnicity |
|---|---|---|---|---|
| 1 | Abdel-Haq et al. (2021) (12) | 33/15 | 6.0 (3.0 - 9.0) | African American (75.8%), Middle Eastern (6.1%), Caucasian (9.1%), other (3%) |
| 2 | Alkan et al. (2021) (13) | 36/19 | 7.8 (1.4 - 17) | NR |
| 3 | Başar et al. (2021) (14) | 24/14 | 9.2 (0.8 - 15) | NR |
| 4 | Belhadjer et al. (2020) (15) | 35/18 | 10 (2 - 16) | NR |
| 5 | Blumfield et al. (2021) | 16/10 | 10 (1.6 - 20) | NR |
| 6 | Capone et al. (2020) (17) | 33/20 | 8.6 (5.5 - 12.6) | Hispanic (27%), non-Hispanic (73%), white non-Hispanic (37%), black non-Hispanic (21%), Asian (15%), and other (24%). |
| 7 | Carter et al. (2020) (18) | 25/15 | 12.5 (7.7 - 14.4) | Asian (20%), black (36%), white (40%), other (4%) |
| 8 | Miller et al. (2019) (19) | 44/20 | 11 (0.6 - 20) | White, non-Hispanic (20.5%), black/African American (20.5), Hispanic (34%), not reported/declined to answer (25%) |
| 9 | Whittaker et al. (2020) (20) | 58/38 | 9 (5.7 - 14) | Black (38%), Asian (31%), white (21%), other (10%) |
| 10 | Davies et al. (2020) (21) | 78/52 | 11 (8 - 14) | Afro-Caribbean (47%), Asian (28%), white (22%), other (3%) |
| 11 | Dufort et al. (2020) (4) | 99/53 | < 21 | Hispanic (36%), non-Hispanic (64%) |
| 12 | Dionne et al. (2020) (22) | 25/15 | 9.7 (2.7 - 15.0) | NR |
| 13 | Pouletty et al. (2020) (23) | 268/161 | 8.21 (4.03 - 12.09) | White (42.5%), black/African/ Caribbean/ black British (22.4%), Asian/ Asian British (23.5%), Mixed/ multiple ethnic groups (4.1%), other ethnic groups (3.7%) |
| 14 | Hameed et al. (2021) (24) | 35/27 | 11 (6 - 14) | NR |
| 15 | Dhanalakshmi et al. (2020) (25) | 19/8 | 6 (1.1 - 16) | NR |
| 16 | de Farias et al. (2020) (26) | 11/9 | 4.9 (0.6 - 11) | NR |
| 17 | Moraleda et al. (2021) (27) | 31/18 | 7.6 (4.5 - 11.5) | NR |
| 18 | Feldstein et al. (2020) (28) | 186/115 | 8.3 (3.3 - 12.5) | White, non-Hispanic (19%), black, non-Hispanic (25%), Hispanic or Latino (31%), another race, non-Hispanic (5%) |
| 19 | Toubiana et al. (2020) (29) | 21/9 | 7.9 (3.7 - 16.6) | NR |
| 20 | Sozeri et al. (2021) (30) | 67/48 | 1 - 18 | NR |
| 21 | Haslak et al. (2021) (31) | 76/22 | 8.17 ± 4.42 | NR |
| 22 | Bowen et al. (2021) (32) | 2818/1664 | < 20 | Hispanic/Latino (37%), non-Hispanic black (28.8%), non-Hispanic white (27.7%) |
| 23 | Al-Harbi et al. (2021) (33) | 54/30 | 3.4 | Arab (75.9%), black or African (7.4%), South Asian (3.7%), Southeast Asian (5.6%), West Asian (7.4%) |
| 24 | Feldstein et al. (2021) (34) | 539/312 | 8.9 (4.7 - 13.2) | White, non-Hispanic (13.3%), black, non-Hispanic (34.7%), Hispanic or Latino (35.9%), other, non-Hispanic (5.5%) |
| 25 | Abrams et al. (2021) (35) | 1080/602 | 8 (4 - 12) | NR |
| 26 | Acevedo et al. (2021) (36) | 78/46 | 7 (1 - 11) | NR |
| 27 | Valverde et al. (2021) (37) | 286/194 | 8.4 (3.8 - 12.4) | White (56.3%), black (20.6%), Asian (10.1%), Mixed (5.9%), other (7.1%) |
| 28 | Deep et al. (2020) (38) | 116/76 | 11 (7 - 14) | Afro-Caribbean (45%), Asian (26%), Caucasian (39%), other (11%) |
| 29 | Tripathi et al. (2021) (39) | 171/103 | 8.7 (5.0 - 13) | Hispanic (27.3%) |
| 30 | Suresh Kumar et al. (2021) (40) | 40/26 | 7 (5 - 10) | NR |
| 31 | Martin et al. (2021) (41) | 439/259 | 9 (1 - 18) | Hispanic (5.69%), non-Hispanic (15.25), white (9.33%), black (7.51%) |
| 32 | Verdugo et al. (2021) (42) | 32/18 | 6.8 ± 3.9 | NR |
| 33 | Kaushik et al. (2020) (43) | 33/20 | 10 (6 - 13) | Hispanic or Latino (45%), black (39%), white (9%), Asian (3%), other (3%) |
| 34 | Lee et al. (2020) (44) | 28/16 | 9 (1 - 17) | White (36%), black (18%), Hispanic (43%) |
| 35 | Lima-Setta et al. (2021) (45) | 56/39 | 6.2 (2.4 - 10.3) | White (41%), mixed race/ethnicity (39%), black (18%), Asian (2%) |
| 36 | Mamishi et al. (2020) (46) | 45/24 | 7 (10 - 17) | NR |
| 37 | Nino-Taravilla et al. (2021) (47) | 26/15 | 6.5 (2 - 10.5) | NR |
| 38 | Patnaik et al. (2021) (48) | 21/13 | 8.5 (2 - 16) | NR |
| 39 | Penner et al. (2021) (49) | 46/30 | 10.2 (8·8 - 13·3) | African-Caribbean (35%), South Asian (24%), other (22%) |
| 40 | Ramcharan et al. (2020) (50) | 15/11 | 8.8 (6.4 - 11.2) | African/Afro-Caribbean (40%), South Asian (40%), Mixed (13%), other (7%) |
| 41 | Roberts et al. (2022) (51) | 50/33 | 9.65 (6.22- 14.0) | White (30%), black (18%), other (24%), Missing/declined to answer (28%) |
| 42 | Swann et al. (2020) (52) | 52/31 | 4.6 (0.3 - 13.7) | White (57%), South Asian (12%), black (10%) |
| 43 | Torres et al. (2020) (53) | 27/14 | 6 (0 - 14) | NR |
| 44 | Rosanova et al. (2021) (54) | 25/9 | 8.6 (5.1 - 10.5) | NR |
Bibliographic and Demographic Data of Articles Identified in the Systematic Review a
Among the patients included in the analysis, 418 (14.8%) individuals reported contact with a confirmed SARS-CoV-2 infection case. Of the patients analyzed, 960 (34.4%) tested positive for SARS-CoV-2 through RT-PCR tests, while 1393 (49.3%) individuals had positive SARS-CoV-2 serology tests. Moreover, 228 (8%) patients demonstrated positive results for both RT-PCR and serology tests, indicating dual confirmation of SARS-CoV-2 infection.
Table 2 illustrates the presenting features reported in all but one study. The predominant presenting symptom was fever, followed by rash, conjunctivitis, and vomiting.
A comprehensive analysis was conducted on children, revealing the presence of various comorbidities in 2441 (33.3%) cases. Among the patients for whom data were available, obesity (27.13%) emerged as the predominant comorbidity. Additionally, several other comorbid conditions were identified but were less prevalent, affecting less than 10% of the patients. These less frequent comorbidities included chronic respiratory disease (6.47%), neurological disorders (2.01%), autoimmune diseases (0.98%), diabetes (0.46%), hematologic disease (0.43%), eczema (0.04%), kidney disorders (0.04%), mitochondrial disorder, and chromosomal abnormalities (0.01%).
| Main Clinical Symptoms | Patients with the Symptom/Patients with Available Data | Percent |
|---|---|---|
| Constitutional | ||
| fever | 1845/1869 | 98.71 |
| Gastrointestinal | ||
| Vomiting | 3730/5348 | 50.89 |
| Diarrhea | 1465/2691 | 19.98 |
| Abdominal pain | 1769/2867 | 24.13 |
| Respiratory | ||
| Cough | 1380/4776 | 28.89 |
| Shortness of breath | 426/1847 | 23.06 |
| Severe respiratory involvement | 162/2818 | 5.74 |
| Pneumonia | 781/2936 | 26.6 |
| Sore throat | 137/604 | 22.68 |
| Neurologic | ||
| Headache | 207/752 | 27.52 |
| Confusion | 58/660 | 8.78 |
| Seizure | 17/539 | 3.15 |
| Meningitis | 123/2898 | 4.24 |
| Encephalopathy | 76/2818 | 2.69 |
| Stroke | 22/2818 | 0.78 |
| Mucocutaneous | ||
| Conjunctivitis | 3013 /5706 | 52.8 |
| Rash | 3113/5636 | 55.23 |
| Dry cracked lips | 219/526 | 41.63 |
| Edema hand/feet | 191/604 | 31.62 |
| Oral mucosal changes | 235/669 | 35.12 |
| Cardiovascular | ||
| Hypotension | 332/4829 | 6.87 |
| Arrhythmia | 660/4829 | 13.66 |
| Coronary artery dilatation/aneurysm | 893/4829 | 18.49 |
| Myocarditis | 477/4829 | 9.87 |
| Myocardial dysfunction | 126/4829 | 2.6 |
| Ventricular dysfunction | 279/4829 | 5.7 |
| Pericardial effusion | 441/4829 | 9.13 |
| Valvular insufficiency | 115/4829 | 2.38 |
| Renal | ||
| Acute kidney injury | 703/3570 | 19.69 |
| Renal failure | 186/3156 | 5.89 |
| Shock | 2316/5231 | 44.27 |
| KD-like presentation | 208/806 | 25.8 |
Main Clinical Symptoms Extracted from Selected Studies
Thirty-four studies reported on cardiac involvement (6505 patients). Echocardiograms were performed in 6230 cases (95.77%). Of those with echocardiograms, 4829 patients (77.51%) were found to have abnormal results. The most prevalent abnormality observed was coronary artery dilatation or aneurysm, accounting for 893 cases (18.49%), followed by arrhythmia in 660 cases (13.66%). ECG outcomes were reported in only 6 out of 46 studies (n = 140, 35.53%). The predominant ECG changes noted were ST-segment elevation (46.42%), prolonged PR interval (14.28%), and bundle-branch block (11.42%).
4.3. Laboratory Evaluation
The laboratory tests revealed abnormal levels of inflammatory, hematologic, and cardiac markers in the studies, with the details presented in Table 3.
| Laboratory Parameters | Patients with Abnormal Level/Patients with Available Data | Percent |
|---|---|---|
| Inflammatory markers | ||
| ↑ CRP | 3042/3849 | 79.03 |
| ↑ ESR | 795/1144 | 6949 |
| ↑ Procalcitonin (ng/mL) | 714/1218 | 58.62 |
| ↑ LDH | 659/1148 | 57.40 |
| ↑ Fibrinogen | 1195/2135 | 55.97 |
| ↑ D-dimer | 2358/3329 | 71.64 |
| ↑ Ferritin | 2788/2794 | 99.78 |
| ↑IL-6 | 541/2196 | 24.63 |
| ↓ Albumin | 909/1414 | 64.28 |
| Hematologic markers | ||
| ↑ Neutrophil | 524/641 | 81.74 |
| ↓ Lymphocyte (%) | 2234/5179 | 43.13 |
| ↑Leucocyte | 539/1014 | 53.15 |
| ↓ platelets | 2651/6100 | 43.45 |
| Cardiac biomarkers | ||
| Troponin | 3120/5278 | 59.11 |
| BNP | 844/2377 | 35.5 |
| proBNP | 2254/5986 | 37.65 |
Laboratory Findings Extracted from Selected Studies
4.4. Therapeutic Management
Regarding the management of MIS-C, therapeutic approaches and supportive care were described in most studies. The mainstay of management for the MIS-C group included intravenous immunoglobulin (IVIG), steroids, and supportive therapy. Intravenous immunoglobulin therapy emerged as the most commonly used medication, administered in 4588 cases (80.73%), followed by steroids in 3730 cases (65.63%) across the majority of studies. Anticoagulation (enoxaparin or heparin) was administered to 883 patients (15.67%), while Aspirin, as antiplatelet therapy, was prescribed to 1073 patients (19.4%). A smaller proportion of patients, approximately 4.5% (n = 250), received other immunomodulators such as Anakinra, Tocilizumab, and Infliximab. Broad-spectrum antibiotics were used in the initial days of hospitalization to address possible sepsis, reported in 916 cases (16.26%). One hundred two patients required vasoactive drugs, and 496 patients (8.8%) needed inotropes for effective management of their condition. Among them, 374 patients (6.6%) required critical care, including intubation or invasive mechanical ventilation. Sixteen patients required extracorporeal membrane oxygenation (ECMO). Convalescent plasma therapy was the least commonly utilized approach, reported in only 12 patients (Table 4).
| Study No | PICU Admission No. | Therapeutic Management | Outcomes | Mortality |
|---|---|---|---|---|
| 1 | 22 | IVIG (n = 29), steroids (n = 27), infliximab (n = 12), aspirin (n = 29), antibiotics (n = 27), antivirals (n = 27) | Mechanical ventilation (n = 9), inotropic support (n = 24), ECMO (n = 3) | 0 |
| 2 | 4 | IVIG (n = 36), methylprednisolone (n = 36), enoxaparin (n = 36), aspirin (n = 36), antibiotics (n = 26) | NR | 8 |
| 3 | 2 | IVIG (n = 11), methylprednisolone (n = 10), enoxaparin (n = 11), aspirin (n = 11), anti-interleukin-1 (n = 2) | Inotropic support (n = 3). | 1 |
| 4 | 35 | IVIG (n = 25), steroids (n = 12), anti-interleukin-1 (n = 3), heparin (n = 23), | Inotropic support (n = 28), non-invasive mechanical ventilation (n = 11), invasive mechanical ventilation (n = 22) | 0 |
| 5 | 11 | IVIG (n = 5), steroids (n = 10), anti-interleukin-1 (n = 2), | Intubation (n = 1), | 0 |
| 6 | 26 | IVIG (n = 33), methylprednisolone (n = 23), anti-interleukin-1 (n = 4), inhibitor of il-6r, tocilizumab (n = 3), infliximab (n = 1), enoxaparin (n = 14), aspirin (n = 29) | Mechanical ventilation (n = 6) | 0 |
| 7 | 21 | IVIG (n = 23), steroids (n = 20), biologic immunomodulation (n = 14) | Mechanical ventilation (n = 12). | 0 |
| 8 | NR | IVIG (n = 36), steroids (n = 42), anticoagulation (n = 40), anti-interleukin-1 (n = 8) | Intubation or invasive mechanical ventilation (n = 1), renal replacement therapy (n = 1) | 0 |
| 9 | 29 | IVIG (n = 41), steroids (n = 37), anti-interleukin-1 (n = 3), infliximab (n = 8) | Intubation or invasive mechanical ventilation (n = 25), inotropic support (n = 27), ECMO (n = 3) | 1 |
| 10 | 78 | IVIG (n = 59), steroids (n = 57), anti-interleukin-1 (n = 8), infliximab (n = 7), tocilizumab (n = 3), rituximab (n = 1), aspirin (n = 45), antibiotics (n = 78), antivirals (n = 1) | Intubation or invasive mechanical ventilation (n = 36), non-invasive mechanical ventilation (n = 5), inotropic support (n = 65), renal replacement therapy (n = 1) | 2 |
| 11 | 79 | IVIG (n=69), Steroids (n=63), Glucocorticoids and IVIG (n=48) | Vasopressor support (n=61), mechanical ventilation (n=10), ECMO (n=4) | 2 |
| 12 | 14 | IVIG (n = 16), steroids (n = 13), anti-interleukin-1 (n = 4), enoxaparin (n = 14), aspirin (n = 14), antivirals (n = 9) | Non-invasive mechanical ventilation (n = 6), inotropic support (n = 7 | 0 |
| 13 | 118 | IVIG (n = 189), steroids (n = 149), aspirin (n = 196), anti-interleukin-1 (n = 10), inhibitor of il-6r (n = 4), antibiotics (n = 249), antivirals (n = 18) | Non-invasive mechanical ventilation (n = 44), inotropic support (n = 80), dialysis (n = 1) | 3 |
| 14 | 24 | IVIG (n = 35), steroids (n = 35) | Mechanical ventilation (n = 35), inotropic support (n = 20), ECMO (n = 2) | 1 |
| 15 | 12 | IVIG (n = 15), steroids (n = 11), aspirin (n = 16), inhibitor of IL-6R (n = 1) | Vasoactive support (n = 6), fluid resuscitation (n = 10) | 0 |
| 16 | 11 | NR | Intubation or invasive mechanical ventilation (n=3), non-invasive mechanical ventilation (n=4), fluid resuscitation (n=8) | 2 |
| 17 | 20 | IVIG (n = 20), steroids (n = 21), both IVIG and corticosteroids (n = 13), antibiotics (n = 3), antivirals (n = 9) | Intubation or invasive mechanical ventilation (n = 6), fluid resuscitation (n = 2) | 1 |
| 18 | 148 | IVIG (n = 144), steroids (n = 91), anticoagulation (n = 14), interleukin-6 inhibitor (n = 14), interleukin-1r inhibitor (n = 24) | Intubation or invasive mechanical ventilation (n = 37), ECMO (n = 8) | 4 |
| 19 | 17 | IVIG (n = 21), steroids (n = 7), aspirin (n = 21), antibiotics (n = 18) | Mechanical ventilation (n = 11), inotropic support (n = 18) | 0 |
| 20 | 21 | IVIG (n = 58), steroids (n = 45), anti-interleukin-1 (n = 17), tocilizumab (n = 1), enoxaparin (n = 42) | Vasoactive medications (n = 14), mechanical ventilation (n = 2), ECMO (n = 2) | 2 |
| 21 | 27 | IVIG (n = 76), steroids (n = 74), anticoagulation (n = 70), anti-interleukin-1 (n = 3), antivirals (n = 7) | Intubation or invasive mechanical ventilation (n = 3), non-invasive mechanical ventilation (n = 9), inotropic support (n = 22), Plasmapheresis (n = 14) | 1 |
| 22 | 1628 | IVIG (n = 33), steroids (n = 14), both IVIG and glucocorticoids (n = 14) | NR | 35 |
| 23 | 54 | IVIG (n = 50), steroids (n = 47), anti-interleukin-1 (n = 17), tocilizumab (n = 8), aspirin (n = 27), enoxaparin (n = 16), macrolides (n = 26), hydroxychloroquine (n = 2), antivirals (n = 21), antibiotics (n = 53) | Mechanical ventilation (n=43), inotropic support (n=32) | 9 |
| 24 | NR | IVIG (n = 15), aspirin (n = 8), anti-interleukin-1 (n = 1), anti-il-6 treatment (n = 1), hydroxychloroquine (n = 1), | Intubation or invasive mechanical ventilation (n = 2), non-invasive mechanical ventilation (n = 3). | 0 |
| 25 | 398 | IVIG (n = 415), steroids (n = 374), anticoagulation (n = 337), aspirin (n = 308), tocilizumab (n = 32), hydroxychloroquine (n = 14) | Convalescent plasma therapy (n = 10) | 10 |
| 26 | 684 | NR | NR | 18 |
| 27 | 78 | IVIG (n = 71), steroids (n = 55), anticoagulation (n = 34), aspirin (n = 34), immunomodulator (eculizumab) (n = 1) | Vasoactive drugs (n = 59), respiratory support (n = 54), dialysis (n = 9) | 7 |
| 28 | NR | IVIG (n = 224), steroids (n = 80), anticoagulation (n = 108), aspirin (n = 212), diuretics (n = 175), antibiotics (n = 202), antivirals (n = 20). | Inotropic support (n = 80) | 1 |
| 29 | NR | NR | Intubation or invasive mechanical ventilation (n = 41), inotropic support (n = 74), vasoconstricted shock (n=), vasodilated shock (n = 57) | 2 |
| 30 | NR | IVIG (n = 78), steroids (n = 115), anticoagulation (n = 39), anti-interleukin-1 (n = 21), antibiotics (n = 13), hydroxychloroquine (n = 5), antivirals (n = 27) | Convalescent plasma therapy (n = 1), intubation or invasive mechanical ventilation (n = 30), non-invasive mechanical ventilation (n = 32), inotropic support (n = 73) | 8 |
| 31 | 34 | IVIG (n = 40), steroids (n = 34), heparin (n = 3), steroids +ivig (n = 34) aspirin (n = 32) | Intubation or invasive mechanical ventilation (n = 9), non-invasive mechanical ventilation (n = 9) fluid resuscitation (n = 26) | 2 |
| 32 | NR | NR | Intubation or invasive mechanical ventilation (n = 12), inotropic support (n = 31) | 0 |
| 33 | 24 | IVIG (n = 24), steroids (n = 21), enoxaparin (n = 24), aspirin (n = 23), tocilizumab (n = 2), infliximab (n = 1) | NR | 0 |
| 34 | 33 | IVIG (n = 18), steroids (n = 17), tocilizumab (n = 7), anti-interleukin-1 (n = 4), anticoagulation (n = 32), antibiotics (n = 15), antiviral (n = 7) | Convalescent plasma therapy (n = 1), Vasoactive medications (n = 17), intubation or invasive mechanical ventilation (n = 5), non-invasive mechanical ventilation (n = 2), ECMO (n = 1) | 1 |
| 35 | 17 | IVIG (n = 22), anti-interleukin-1 (n = 5), steroids (n = 17) | Non-invasive mechanical ventilation (n = 7) | 0 |
| 36 | NR | IVIG (n = 50), steroids (n = 31), enoxaparin (n = 29), aspirin (n = 25), antibiotics (n = 33), antivirals (n = 5) | Intubation or invasive mechanical ventilation (n = 6), non-invasive mechanical ventilation (n = 3) | 1 |
| 37 | NR | IVIG (n = 18), steroids (n = 27) | 5 | |
| 38 | NR | IVIG (n = 16), steroids (n = 18), tocilizumab (n = 3), infliximab (n = 1) | Vasoactive drugs (n = 13), intubation or invasive mechanical ventilation (n = 10). | 0 |
| 39 | 10 | IVIG (n = 7), steroids (n = 20), enoxaparin (n = 20), | Intubation or non-invasive mechanical ventilation (n = 5) | 0 |
| 40 | NR | IVIG (n = 38), steroids (n = 25), enoxaparin (n = 35), anti-interleukin-1 (n = 6), | Mechanical ventilation (n = 16), inotropic support (n = 22) | 0 |
| 41 | 10 | IVIG (n=10), Methylprednisolone (n=5), Aspirin (n=11), Antibiotics (n=15) | Intubation or invasive mechanical ventilation (n=10), non-invasive mechanical ventilation (n=4), respiratory support (n=4) | 0 |
| 42 | 25 | NR | Mechanical ventilation (n = 2), ECMO (n = 1) | 0 |
| 43 | 38 | IVIG (n = 28), steroids (n = 24), anti-interleukin-1 (n = 3), tocilizumab (n = 1), adalimumab (n = 1), infliximab (n = 1), antibiotics (n = 186), antivirals (n = 38). | Intubation or invasive mechanical ventilation (n = 14), non-invasive mechanical ventilation (n = 18), inotropic support (n = 25) | 0 |
| 44 | 16 | IVIG (n = 19), steroids (n = 17), enoxaparin (n = 18), aspirin (n = 17), antibiotics (n = 24), | Intubation or invasive mechanical ventilation (n = 12), oxygen (n = 13), vasoactive drugs (n = 12) | 0 |
Therapeutic Management and Outcomes Extracted from Selected Studies
4.5. Outcomes
All patients with MIS-C were hospitalized. Out of the total 7313 patients, 3784 (51.74%) required admission to the pediatric intensive care unit (PICU), with a median duration of 4.7 days (2 - 18 days). Among MIS-C cases, 127 deaths were reported, accounting for 1.7% of the total patients. However, it is crucial to note that not all fatalities were directly attributed to MIS-C (Table 4).
5. Discussion
Multisystem inflammatory syndrome in children is a consequence of an amplified innate and adaptive immune response, characterized by a pronounced cytokine storm. This heightened immune reaction is presumed to be activated in susceptible children following prior exposure to SARS-CoV-2. The manifestation of MIS-C thus reflects the intricate interplay between the immune system and the viral pathogen, contributing to a more nuanced understanding of the syndrome's pathophysiology (55). This systematic review analyzed and summarized 44 published studies involving 7297 patients with MIS-C. While specific case definitions vary, definitions for MIS-C have been proposed by the CDC (56), the World Health Organization (WHO) (57), and the Royal College of Pediatrics and Child Health (RCPCH) (58).
Most of the included studies (32 studies) used the CDC clinical definition for MIS-C, while 12 adopted the WHO definition (six of which incorporated both CDC and WHO criteria). Additionally, seven studies employed the RCPCH definition (four using either CDC or RCPCH criteria), and two did not specify the criteria used. MIS-C can affect children at any age, ranging from infancy (< 1 year) through late adolescence. The exact pathophysiology of the disease remains elusive due to the lack of precise data. Children with COVID-19 often display milder symptoms and are less frequently tested than adults.
Clinical laboratory testing has played a crucial role in managing SARS-CoV-2. While PCR-based assays have proven robust for virus detection, serology tests are also valuable as a complementary approach. In this review, all studies except for five (12, 32, 34, 35, 42) reported results of SARS-CoV-2 RT-PCR or serology tests.
In numerous systematic reviews of MIS-C, elevated levels of inflammatory markers such as CRP, ESR, procalcitonin, LDH, fibrinogen, D-dimer, ferritin, and IL-6 have been observed. This finding aligns with the results of several studies included in our research (59, 60). The precise factors driving this inflammatory response remain unclear, but it appears that infection with the virus triggers an immune system reaction.
It is crucial to acknowledge that COVID-19 tends to be more severe in patients with underlying medical conditions (61). In our review, the majority of children diagnosed with MIS-C had no pre-existing medical conditions, with obesity being the most frequently observed underlying condition (27.40%). Some studies have reported comorbidities with a frequency ranging between 20% and 25%. Apart from obesity (28, 52), other comorbidities were infrequent, primarily comprising respiratory diseases such as asthma and chronic lung disease, cardiovascular diseases, and immunodeficiencies (4, 20, 28, 44). In a systematic review conducted by Kaushik et al., it was revealed that 23.3% of children exhibited comorbidities (43).
The findings in the current review confirm the diverse range of clinical manifestations, from mild to severe involvement. Fever was the most frequent symptom, followed by rash, vomiting, and conjunctivitis. In most reports, fever and gastrointestinal symptoms such as abdominal pain, diarrhea, and vomiting are described as the most common (60, 62). This contrasts with adults with COVID-19, where respiratory symptoms predominate (63). A significant portion of children in this review either did not exhibit respiratory symptoms (70%) (14, 20, 24, 28, 36, 41, 44, 47, 50, 52) or presented with milder manifestations.
Cardiac manifestations stand out as predominant symptoms in children with MIS-C. In suspected MIS-C patients, a complete cardiac evaluation should be conducted, including assessment of troponin and BNP levels, ECG, and echocardiogram. According to the findings of published studies, cardiac involvement is frequently detected in 67 – 80% of children across a spectrum of severity (34, 64). Based on our review, coronary artery dilatation or aneurysm was observed in 18.49% of cases, followed by arrhythmia in 13.66% of cases and ventricular dysfunction in 5.7% of cases. Notably, the frequency of ventricular dysfunction (34, 37, 49) and arrhythmias (22, 62, 65) in patients with MIS-C was lower than reported in several other studies (66, 67). During our review, we observed significant abnormalities in cardiac markers, including troponin, BNP, and proBNP.
Regarding cardiac involvement, 34 studies involving 4,829 patients were analyzed. Among these, 208 patients from 15 studies met the KD criteria (12, 14, 17, 18, 20, 22, 23, 25, 27, 29-31, 36, 42). Although MIS-C and Kawasaki disease share similar symptoms and characteristics, various reports have highlighted significant differences, such as age, that distinguish the two conditions (57, 68). Studies have shown that MIS-C typically affects older children, whereas KD has been reported in younger children under five (69-72). In our systematic review, the median age of patients with MIS-C was 8.6 years. Age (> 5 years) represents a significant risk factor associated with more severe outcomes in MIS-C (61, 73, 74).
In their study, Kaushik et al. documented cardiac involvement in MIS-C patients exhibiting a Kawasaki-like syndrome, with 20% displaying coronary artery dilatation/aneurysms and hypotension, and 28% experiencing shock (75). In a variable proportion of MIS-C patients, hypotension and shock develop due to acute myocardial dysfunction. Our review found that hypotension occurred less frequently (4, 12, 16, 23, 25, 27, 30, 44, 51), but the incidence of shock (4, 13, 15-17, 19, 20, 22, 23, 26, 27, 29, 32, 37-39, 42, 45, 48) was comparable to other reviews (76-78).
Multisystem inflammatory syndrome in children patients are treated with various regimens. It should be noted that the primary treatment goals for MIS-C involve controlling systemic inflammation, supporting organ function, and reducing the risk of long-term complications (79). Intravenous immunoglobulin and/or steroids are the recommended initial treatment options for diagnosed MIS-C patients (37). While specific studies have indicated the benefits of combination therapy, there are currently no controlled trials directly comparing the efficacy of IVIG and corticosteroids administered alone or in combination. A study of 111 MIS-C patients found a lower treatment failure rate in those who received combination therapy compared to IVIG monotherapy (80). Similarly, another study focusing on MIS-C patients reported that initial treatment with IVIG and steroids was associated with a reduced risk of cardiovascular dysfunction (81). In our review, similar to previous reports, nearly all MIS-C patients received IVIG, with some also receiving combination therapy with steroids (27, 28, 40).
Less than 20% (18.08%) of patients received other immunomodulators, such as anakinra, tocilizumab, and infliximab. Aspirin and anticoagulants are commonly used medications in MIS-C. Given the potential overlap of MIS-C with severe bacterial infection, an initial approach involving empiric broad-spectrum antibiotics should be considered (82). However, it is essential to acknowledge that this approach may only be appropriate for certain patients (83). Recent studies suggest that antiviral therapy may reduce disease severity in some patients (84).
During our review, a significant proportion of MIS-C patients, comprising more than half of the cases, required admission to the PICU. Among those admitted, 53.9% exhibited shock symptoms, with 6.76% requiring intubation or invasive mechanical ventilation, 3.1% requiring non-invasive mechanical ventilation, and 3.7% succumbing during their PICU stay. Multisystem inflammatory syndrome in children emerged as the primary reason for PICU admission associated with COVID-19 infection in Spain. Barrio's study noted that most patients presented with shock necessitating vasoactive drugs, with only a minority requiring ventilation (85).
Overall, in our review, the majority of patients studied experienced favorable outcomes, and all but 127 were discharged home in stable conditions. However, limited reports provided post-hospital discharge follow-up data. Mortality was lower compared to similar studies, such as the Colombian MISCO study (1.7% vs. 9%) (39). Variability in mortality rates among countries and studies may stem from several factors, including disparities in healthcare access and delays in early diagnosis. De Farias et al. identified a high prevalence of preexisting medical conditions and immunosuppression, which could contribute to the elevated mortality observed in MIS-C (26).
While our systematic review aimed to synthesize data on the epidemiological characteristics of MIS-C patients, providing accurate statistics on the incidence of MIS-C across different racial and ethnic groups was limited by the heterogeneity and incomplete reporting of race/ethnicity data in the included studies. However, investigations in some original studies suggest that race and ethnicity may influence the risk and burden of MIS-C. For example, Javalkar et al. found that Hispanic ethnicity and Black race independently increased the risk of MIS-C, even after accounting for socioeconomic factors (86). Similarly, Stierman et al. reported a higher frequency of MIS-C among Hispanic and non-Hispanic black children compared to the general pediatric population, while non-Hispanic White and non-Hispanic Asian children exhibited a lower frequency (87). These findings underscore the potential impact of race and ethnicity on MIS-C susceptibility and manifestation, potentially driven by genetic, socioeconomic, or environmental factors. Further research with standardized data collection and larger cohorts is necessary to clarify the extent of these disparities and their underlying mechanisms.
This systematic review comprehensively summarized the clinical features, management, and outcomes of patients with MIS-C across 44 global studies. Our search process was conducted meticulously, though we acknowledge the possibility of unintentionally overlooking certain studies. To maintain the integrity of our findings, we excluded studies or patients with insufficient data or inadequate descriptions of MIS-C presentations. Specifically, our review did not include case reports, case series, or letters to the editor lacking detailed descriptions of pediatric MIS-C.
However, our study had some limitations worth noting. Firstly, our ability to gather sufficient data for robust analysis was hindered by the retrospective nature of some included studies and incomplete reporting of all variables. Consequently, formal statistical testing could not be conducted in this systematic review. Secondly, the absence of mean and standard deviation (SD) in certain studies limited our ability to directly perform meta-analysis, as we only had access to median, interquartile range (IQR), or range values. A third limitation was the exclusion of studies with sample sizes of fewer than 10 patients. Despite the substantial number of published MIS-C studies, we aimed to synthesize data from sufficiently large cohorts to draw generalizable conclusions. However, this exclusion criterion may have overlooked valuable data from smaller case series, potentially limiting the comprehensive capture of rare manifestations. While this approach aimed to enhance data accuracy by minimizing false positives, it might have unintentionally discounted critical clinical findings described solely in smaller studies. The significant heterogeneity among published MIS-C studies posed challenges for analysis, necessitating exclusions to effectively synthesize current literature. Future reviews could explore these smaller studies to extract additional details on atypical presentations indicative of severe disease courses. Finally, the novelty of MIS-C as a disease has resulted in a scarcity of post-discharge outcome studies, limiting our ability to thoroughly explore the underlying mechanisms of MIS-C.
5.1. Conclusions
Despite its limitations, this systematic review, involving 7,297 patients, provides crucial insights into the diverse clinical manifestations of MIS-C, underscoring the importance of prompt recognition and comprehensive evaluation. Intravenous immunoglobulin and corticosteroids emerged as mainstay therapies, yet evidence-based guidelines for optimal treatment combinations are needed. Vigilant monitoring and multidisciplinary care, including critical care support, are crucial, given that many patients required intensive care. While short-term outcomes were favorable, the long-term implications remain unknown, highlighting the necessity for ongoing follow-up and surveillance studies.