1. Context
Human papillomavirus (HPV) infection is extremely common throughout the world, and so far, 70 types of it have been identified (1-3). In most people, they do not cause any problems, but some types can cause genital warts or cancer. However, sometimes the virus can cause painless growth or lumps around the vagina, penis, or anus. Depending on the oncogenic potential, some HPV types are categorized as low-risk HPV (HPV6 and 11) and high-risk HPV (HPV16, 18, 31, and 33) (4). Hopefully, some HPV vaccines that show strong protection against cervical infections will be introduced (5-7). There are great studies on HPV infections (8), and in this review, HPV infection and new treatments are highlighted, including pathogenesis, virus characteristics, HPV vaccine, loop-mediated isothermal amplification (LAMP), HPV lateral flow dipstick (LFD) tests, and LAMP-LFD in HPV assay (9). Human papillomaviruses are the primary cause of cervical cancer and certain head and neck cancers, such as oropharyngeal squamous cell carcinoma and sinonasal carcinoma. Cervical cancer is commonly diagnosed using liquid-based cytology, followed by HPV testing via commercially available DNA polymerase chain reaction (PCR), p16 immunohistochemistry (IHC), or DNA/RNA in situ hybridization. In head and neck cancers, HPV is typically diagnosed using p16 IHC or RT-q PCR of HPV16 E6 and E7 oncoproteins (10). Several methods exist for collecting oral specimens, as well as PCR-based methods for detecting oral HPV infection. These are used in epidemiological and clinical studies to diagnose mucosal HPV infection. However, it is suspected (though not proven) that these various sample methods and techniques differ in performance, sensitivity, and HPV type specificity (11). This review considers how new knowledge of LAMP can be used to improve vaccine technologies and provide such preventive strategies to maximize the reduction of HPV-related diseases.
2. Evidence Acquisition
2.1. Virus Characteristics
The papillomavirus is a non-enveloped capsid with 50 nm in diameter. Papillomavirus DNA is a double helix from 5748 to 8607 nucleotides. Papillomaviruses infect reptiles, birds, fish, and mainly mammals (12). Their genomes are divided into 2 groups of open read frames (ORFs) that are designated as E or L and upstream regulatory regions (URRs) (13). Core genes L1, E1, E2, and L2 during the virus life cycle have essential functions (14, 15). E7, E6, and E5 genes are not present in all PV types and have evolved to facilitate viral replication in the squamous epithelium (15). The upstream regulatory region is where viral replication originates and serves as a location for cellular and viral transcription factors. Within infected cell nuclei, the E1 protein plays a crucial role in amplifying and replicating the viral genome. The E2 protein acts as a transcription factor and controls the viral lifecycle. The L1 protein is the primary creator of the viral capsid (16, 17). The L2 protein is involved in the encapsidation of DNA. The E4 viral protein binds to cytokeratin and has some characteristics of the core proteins (16, 17). The E5 protein is involved in apoptosis and evading the immune response. Proteins E6 and E7 play roles in amplification and carcinogenesis (12). During evolution, papillomaviruses have evolved about the host species, having high compatibility and cellular specificity for epithelial tissues (18). Human papillomaviruses have 5 genera: Alpha-PVs with 14 classified species and 65 types that produce macroscopically visible lesions; beta-PVs with 6 classified species and 52 types that produce subclinical infections; gamma-PVs with 27 classified species and 97 types that produce subclinical infections in childhood; mu-PVs with 3 species and 3 types; and nu-PVs (-HPV) (18).
2.2. Human Papillomavirus Pathogenesis
The human papillomavirus is a very widespread infection, although most infected people eliminate evidence of the virus without causing known clinical manifestations (19). Most sexually active adults, at some point in their lives, will have an HPV infection. This public health problem has differences based on the geographic region, age, and population. The human papillomavirus is associated with cancers such as anal and head and neck squamous cell carcinoma (HNSCC) (20). Thus, only a small number of individuals infected with HPV go on to develop invasive cervical cancer. Previous research has categorized the risk of developing cervical cancer as undetermined, high-risk, probable high-risk, and low-risk. Humans serve as the sole natural reservoir for HPV and other members of the PV family that affect various species. The human papillomavirus transmission most commonly occurs during penile, vaginal, anal, or oral sexual activity. Key risk factors for sexually transmitted infections include youth, sexual activity, and gender. Some studies indicate that infection can occur after the first sexual encounter. A prior study estimated that in the United States, the prevalence of active HPV infection, HPV-causing cytological abnormalities, and HPV-causing genital warts in the population was 10%, 4%, and 1%, respectively (21).
2.3. Human Papillomavirus Vaccines and Their Mechanisms
Primarily, HPV infections are transmitted through skin-to-skin or skin-to-mucosa contact. Mucosal HPV types are classified according to their oncogenic potential. They are categorized as either low-risk HPV/non-oncogenic types, such as HPV11 and 6, which are commonly associated with warts, or high-risk HPV/oncogenic types, including HPV 18, 33, and 16 (22). Gardasil (quadrivalent HPV vaccine) is the first commercially available HPV vaccine since 2006 (23). Cervarix (bivalent HPV vaccine) was approved in 2009 (24). In 2014, Gardasil 9 (9-valent) vaccine was licensed by the US Food and Drug Administration (FDA) (25). Overall, in many regions, national HPV programs cover a low percentage of the global target population, and the dose coverage is low (26). In high-income countries, HPV coverage is more than 60%, such as Australia, Denmark, and Sweden, and about 32% of females aged 10 - 20 years received the full-dose vaccination (27, 28). Most low-income countries remain unprotected, and in low-income countries, less than 2% of adolescent girls receive a full course of the HPV vaccine (26). In middle- and low-income countries, this gap also exists between the rural and urban residents. In these countries, more than 80% of cervical cancers lead to death (29). In these countries, this statistic shows the necessity of implementing the HPV vaccine to improve public health (27).
Currently, based on a virus-like particle (VLP) of the protein L1, HPV vaccines are developed (30). Virus-like particles can be produced in yeast, insect cells, and bacteria. Since VLPs do not contain a viral genome, these HPV vaccines are considered non-infectious and non-oncogenic (31). Cervarix comprises HPV18 and 16 VLPs and monophosphoryl lipid A (MPL) (32). Monophosphoryl lipid A can induce high antibody levels, and Gardasil contains VLPs against HPV6, 18, 16, and 11 (33). L1-VLPs-based HPV vaccines neglect many other genotypes of oncogenic HPV and provide type-restricted immunity. Because of these limitations, chimeric L1-L2 VLP and L2-VLP, for their broader genotype coverage, are receiving a lot of attention (34, 35). L2-VLP contains type-common epitopes. This structural feature provides broad cross-neutralizing antibody responses (7). Cervarix induces high anti-HPV18 and 16 antibody titers, demonstrating robust protection against oral infections caused by HPV18 and 16 (36). This vaccine invokes long-term and high cross-reactive immunogenicity against HPV45 and 31. Cervarix protects against vaccine-targeted HPV-related abnormalities (37). Gardasil shows suitable efficacy against cervical cancer genital warts, precursor lesions, and cervical HPV infection (5). On the other hand, in the penis, anus, and vulva, this vaccine decreases HPV infections (38). However, this vaccine demonstrated less cross-protection efficacy than Cervarix (39). Gardasil 9 also showed a very high percentage of inhibition in the incidence of vaginal and vulvar diseases. A previous study showed that antibodies induced by Gardasil 9 by transferring across the placenta protected the infant from HPV11 and 6 infections (40). Since HPV types are not covered by the vaccine, Gardasil 9 has little effect on infections and diseases and has low cross-protective efficacy besides the 9 vaccine types (41). A systematic meta-analysis showed that vaccines reduced the prevalence of HPV-related endpoints, and HPV18 and 16 decreased significantly (42).
The optimal time for HPV-related disease prevention is before HPV exposure. Vaccination prior to the onset of sexual activity can offer enhanced protection against HPV infections. However, after sexual contact, vaccination against HPV only offers protection against slightly more than half of the infections (43). For cervical cancer, the most cost-effective solution is vaccinating 12-year-old girls. However, HPV types and vaccine efficacy differ in cancer, and vaccine efficacy varies by geographical region (44). Previous studies have shown that all 3 vaccines exhibit tolerance and safety (45). Gardasil is immunogenic, clinically effective, and well-tolerated in preadolescents and adolescents (46). Furthermore, after vaccination, Gardasil 9 and Cervarix show great antibody nourishment and tolerance (46). Cervarix can also lead to vomiting, dizziness, diarrhea, fever, myalgia, and nausea (47). The HPV vaccination period overlaps with post-vaccination symptoms (41, 48). However, more effort is needed to adjust the components of these vaccines.
2.4. Human Papillomavirus Molecular Detection Methods: Loop-Mediated Isothermal Amplification
All cases of cervical cancer have been attributed to high-risk HPV infections, and among these genotypes, HPV16 to 18 are prevalent. For HPV18 and HPV16 detection, many types of tests are available that detect the high-risk HPV DNA in patient samples. However, these methods have limitations as they do not identify specific HPV genotypes and do not detect non-amplified HPV DNA (49). For HPV genotype detection, the PCR method offers high specificity and sensitivity (50). For HPV screening, different methods are available, such as PCR hybridizations, cytologic evaluations, PCR, and real-time PCR. Real-time PCR is one of the revolutionized molecular diagnostic methods that is used as a routine diagnostic assay for rapid detection and genotyping of clinical samples. Recently, an in-house HPV typing assay using multiplex real-time PCR for molecular epidemiology surveys and diagnosis was introduced in Iran (51). On the other hand, for these methods, special devices are required, which are costly and time-consuming. All life science fields, such as clinical medicine, genetic traits, and genetic disorders, have particularly benefited from PCR techniques (52). In addition, some other amplification techniques have been invented. They include self-sustained sequence replication (3SR) (53), strand displacement amplification (SDA) (54), and nucleic acid sequence-based amplification (NASBA) (55). Each of these methods has its own innovation. For example, PCR uses heat denaturation of DNA, but the NASBA and 3SR use a set of reverse transcription reactions that eliminate heat denaturation in the target sequence amplification (56). Furthermore, the use of a restriction enzyme in SDA eliminates the need for heat denaturation (56). These methods can amplify DNA but still have problems (57). Due to low specificity for the selection of target sequence, these methods require amplification of a precision instrument or employ elaborate methods for the detection of the amplified products. Despite the simplicity and achievable amplification size, the need for a thermal cycler prevents the widespread use of this powerful method.
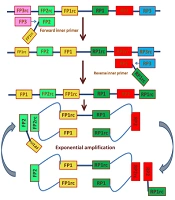
Furthermore, although 3SR and NASBA do not use thermal cycling, these methods are compromised in specificity. Strand displacement amplification does not have this limitation by using isothermal conditions and 4 primers, but due to the digestion of irrelevant DNA and the costly modified nucleotides, the backgrounds increase. Although in nested PCR and SDA, the use of multiple primers has improved the specificity of amplification, the irrelevant sequences co-amplification still remains a challenge. Recently, a novel method called “loop-mediated isothermal amplification” has been developed. This method offers enhanced specificity and the ability to amplify even a few DNA copies. The LAMP method has been developed for HPV genotyping using turbidity (58). In the LAMP method, by repetition of 2 types of elongation reactions, gene amplification proceeds and occurs via the loop regions. In addition, a set of different primers binds to 6 or 8 regions on the target gene (56). These 6 different primers consist of 2 inner primers (FIP and BIP), 2 outer primers (F3 and B3), and loop primers (loop forward and backward). The elongation reactions are sequentially repeated using the stem-loop regions. The LAMP reaction was started using Bst DNA polymerase in an isothermal condition (Figure 1) (59).
Loop-mediated isothermal amplification is based on auto-cycling strand-displacement DNA synthesis using a DNA polymerase and 2 pairs of primers (60)
The LAMP reaction differs from other amplification methods, such as a high specificity characteristic of the inner primer, utilization of 6 gene regions for amplification, the constant temperature, and completion of amplification within 1 h (61). Notably, LAMP is capable of amplifying small quantities of a gene within a short time. The detection methods, such as turbidity (62) and fluorescence detection (63), can be used because of the large output and high specificity. Both methods allow for visual inspection and real-time detection. According to the characteristics of LAMP, this method could be used in genetic testing in clinical settings, point-of-care testing, and food products and environmental samples testing (61). Loop-mediated isothermal amplification diagnostic applications are beneficial due to their specificity, reading ease of LAMP production, amplification efficiency, and simplicity. Loop-mediated isothermal amplification is conducted at 65°C and can be performed in a cup of warm water, producing over 108 products in 15 - 60 min (64).
2.5. Loop-Mediated Isothermal Amplification and Human Papillomavirus
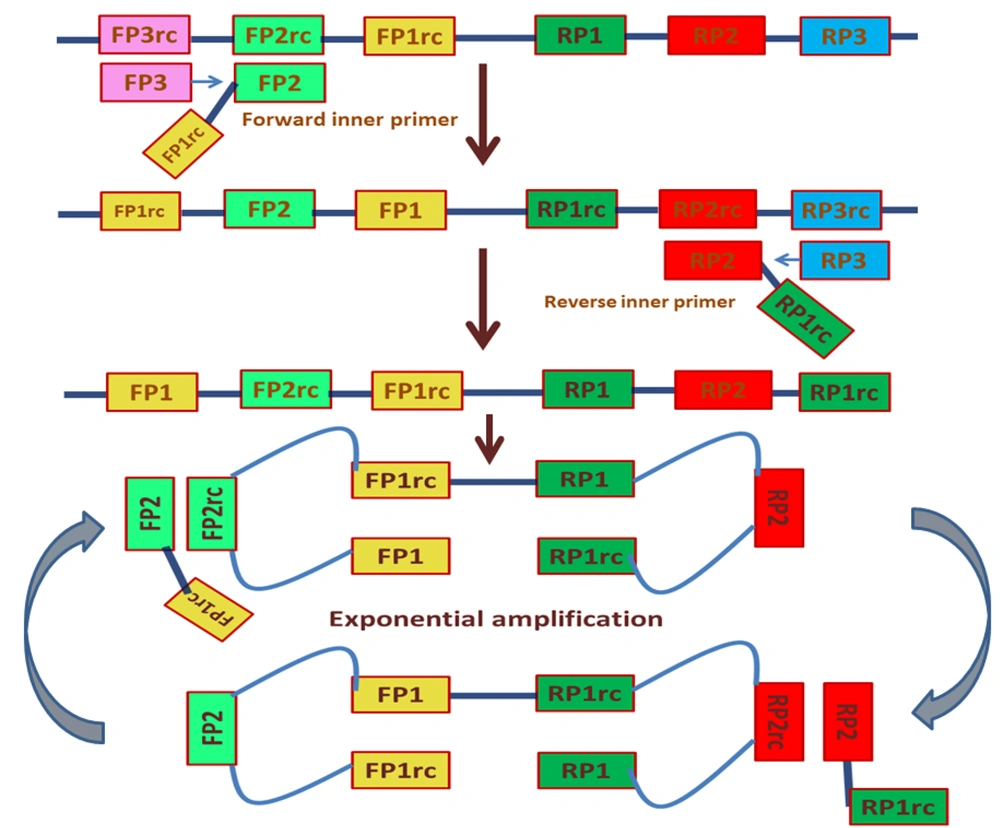
The LAMP technique has been employed in the detection of HPV DNA using various strategies, including quartz crystal microbalance, lateral dipsticks, agarose gel electrophoresis, turbidity measurements, gold nanoparticles, amperometry, pH indicators fluorescent dyes, and self-partitioning droplets (Figure 2) (58, 65-68).
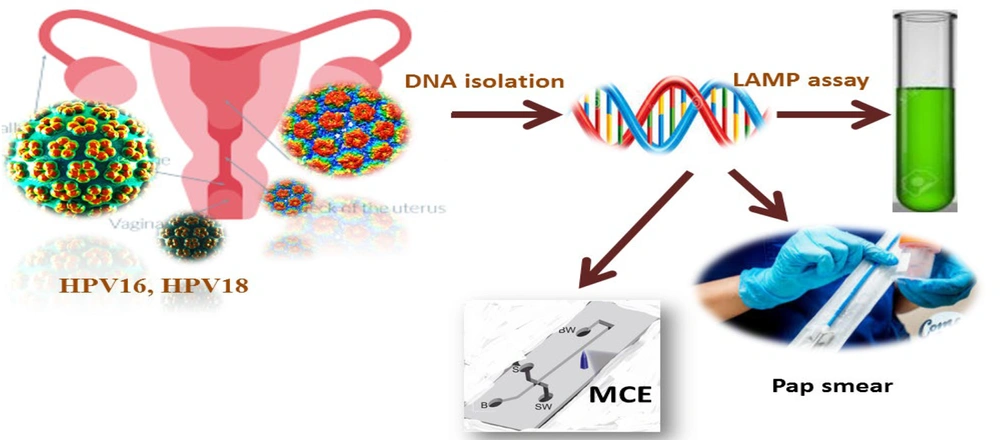
Detecting human papillomavirus (HPV)18 and HPV16 samples with polymerase chain reaction (PCR)-MCE, visual lamp assay, and cytology methods (69)
Some HPV detection methods have been developed, including nucleic acid amplification-based assays, fluorescence in situ hybridization, signal-amplification assays, p16 expression, and nucleic-acid hybridization assays (70). However, these methods require trained personnel, specialized reagents, the expense of the test, and sophisticated equipment, which can be limited to scale-up for the screening, detection, and monitoring of treatment response. Previously, the LAMP technology was developed for the detection of HPV18 and HPV16, and it was used in the clinical setting for the facile, rapid, and cost-effective detection of HPV16 and HPV18 (71). The possible mechanism of HPV infection is described in Table 1.
| Methods | Advantages | Disadvantages |
|---|---|---|
| HPV PCR | High sensitivity, cost-effective | Low specificity, no quantitative measure of viral load, no confirmation of transcriptionally active virus, easy to cross-contamination between samples, high false positive rate, and performing HPV typing operations is tedious |
| IHC | Virus antigen detection, easy to perform, and locate | False-negative findings and low sensitivity |
| Liquid-based assays | Highly sensitive | Limited experience |
| RNA ISH | High sensitivity, high specificity, and to some extent, it can be used as the gold standard for HPV-related tumors | The experimental operation requires high experience and poor repeatability |
| Western blot analysis | NA | NA |
| Southern blotting assay | High specificity, ability to differentiate between episomal and integrated DNA | Not easily applied to FFPE samples |
| RT-PCR p16 immunostaining | High sensitivity, easy and accessible to most laboratories, markers of transcriptionally active virus | Time-consuming, technically difficult, and low specificity |
Common Detection Methods for Human Papillomavirus
However, LAMP has encountered limitations in both the POC and clinical diagnostic settings. These limitations include (1) maintaining a controlled environment for designing LAMP-based microfluidic devices; (2) requiring well-experienced personnel for fabricating integrated LAMP-based chips; (3) automating LAMP before and after the completion of the reaction; (4) choosing primer sets; and (5) accurately diagnosing closely related species among the more complex foodborne pathogens. Additionally, maintaining suitable aspects, such as primer selectivity and other operational conditions, may be challenging.
2.6. Lateral Flow Dipstick Tests
Considering that it is hard to observe turbidity for low-copy DNA identification with the naked eye (72), LFD tests are used for the detection of bacteria, infectious agents, and viruses (73). At first, the LFD technique was called “sol particle immunoassay” (SPIA) (74). Lateral flow assays (LFAs) are important for diagnostic purposes, such as contamination or infection with specific pathogens, abuse of drugs, ascertaining pregnancy, failure of internal organs, food toxicity, and feed (73). They are specifically designed to be outside the laboratory where an on/off signal is sufficient, and results are obtained within 10 - 20 min (73).
2.7. Loop-Mediated Isothermal Amplification-Lateral Flow Dipstick in Human Papillomavirus Assay
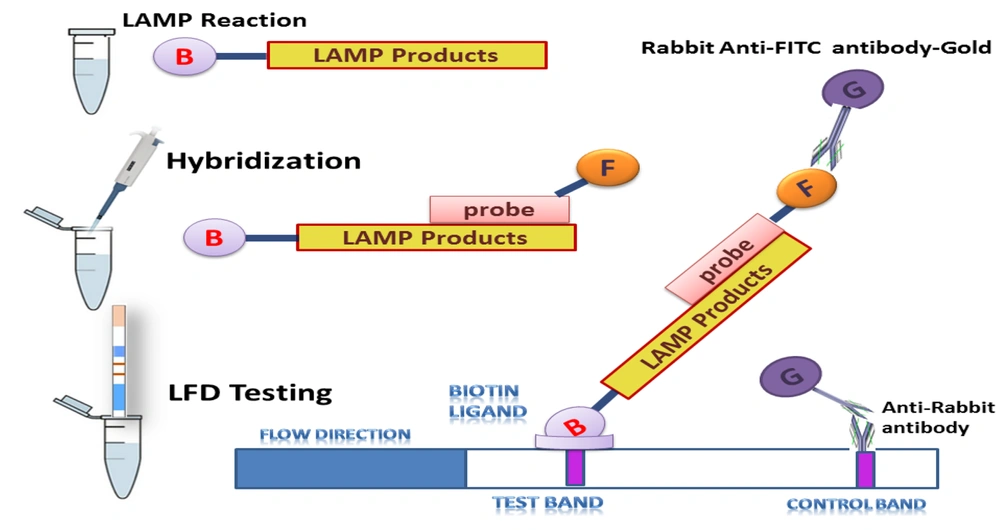
Lateral flow dipstick tests are easy and do not need external instrumentation (75). The combination of LAMP and LFD methods was used to develop an HPV16 and HPV18 assay with high specificity and sensitivity. Loop-mediated isothermal amplification is a method for nucleotide amplification that is simple, fast, and inexpensive, visualizing products using the naked eye (76). However, nonspecific and specific products and false-positive results cannot be separated using the LAMP. Therefore, the LAMP products were hybridized to specific probes and detected by LFD (77). For hybridized LAMP product detection, LFD is sufficient, and without the use of carcinogens, the results are easily read (77, 78). Loop-mediated isothermal amplification has been developed for HPV18 and HPV16 using gel electrophoresis and visual turbidity (14). In another study, with identical LAMP primer sets, the LAMP-LFD combined detection in a shorter time provided higher sensitivity (Figure 3).
The loop-mediated isothermal amplification-lateral flow dipstick (LAMP-LFD) assay design for the detection of human papillomavirus (HPV)16- and HPV18-LAMP products (77)
The detection limits of LAMP-turbidity for HPV18 and HPV16 were 101 and 103 copies, respectively (79, 80). The detection limits of LAMP-LFD for HPV18 and HPV16 were 100 and 101 copies, respectively, in 45 min (79, 80).
3. Discussion
The interpretation of LFD tests is easy and does not need external instrumentation (81). However, HPV has a high diversity, and the HPV type is very important in the rate of cervical carcinogenesis (82). So far, more than 400 types of PV have been identified, and about 18 types have caused infections in humans (83). Papillomaviruses are genetically stable and, at the viral cycle, encode certain proteins with specific functions. Viral proteins are expressed differently during the virus life cycle. The human papillomavirus, by evasion of the host’s immune system, produces clinical infections and participates in the tumor's carcinogenesis (18). So far, the HPV vaccines that were introduced are showing high protection against cervical infections (38, 84). These vaccines have the potential to protect against cancerous HPV. In middle- and low-income countries, more than 80% of cervical cancer deaths occur, showing the necessity of implementing the HPV vaccine to improve public health (85). Virus-like particles that do not contain viral genomes are the basis of today's vaccines (86). These non-oncogenic and non-infectious HPV vaccines can be produced in different living organisms. Gardasil contains VLPs, and MPL in Cervarix can induce the expression of antibodies (87). The L1-VLPs-based HPV vaccines provide type-restricted immunity. Chimeric L1-L2 VLP was developed in this context and provided a broad cross-neutralizing antibody response (7). While there were no significant adverse effects associated with HPV vaccination, further research is necessary to modify the components, like adjuvants, to reduce the adverse effects without compromising the vaccine's effectiveness. Additionally, vaccination before the first sexual contact, as opposed to vaccination after HPV exposure, may provide greater protection against targeted HPV-related infections (43). The tests that are available for the detection of these 2 genotypes detect the presence of high-risk HPV DNA have some limitations (49). For target amplification, PCR using oligonucleotide primers is the most prominent method to amplify DNA. For HPV genotype detection, this method offers high specificity and sensitivity (50). On the other hand, for these methods, special devices are required, which are costly and time-consuming. Genetic disorders are particularly beneficial from PCR. In addition (52), other techniques, such as 3SR, SDA, and NASBA, with their innovation for DNA synthesis, have been invented (53-55). These methods have a good detection limit but still have problems, such as low specificity for the selection of target sequences and high-precision thermal cyclers that prevent the use of these methods (57). Because of the importance of HPV in cancers, as the HPV16 accounts for 87% of oropharyngeal cancers, improved detection of HPV is critical for the rapid detection of cancer, screening of high-risk populations, and monitoring of patients' treatment (71). Recently, a LAMP method was developed for HPV genotyping detection that differs from other amplification methods (58). In the LAMP reaction, within a short time, elongation reactions occur via the loop regions by a set of 4 or 6 different primers. Detection methods such as fluorescence and turbidity allow for real-time detection that can be used in point-of-care and genetic testing. The LAMP reaction was performed without the need for a special reagent and in a general molecular biology laboratory, and reports of the use of this research method have been published. Economically, LAMP is quite cost-effective and inexpensive.
4. Conclusions
Many of these studies relied on separate DNA purification steps. This DNA purification step is a critical step in LAMP and is complicated regarding time, reagents, cost, additional equipment, and expertise that may not be advantageous. On the other hand, LAMP was used in the HPV DNA detection without DNA purification, which reduced the sample processing complexity (88). However, false-positive results cannot be distinguished in LAMP detection methods. Conversely, LFD tests are designed to detect infectious agents and do not require external instrumentation. In this context, LAMP-LFD methods involve hybridizing LAMP products to specific probes and detecting them by LFD for the assay of HPV16 and HPV18 (14). Comparatively, LAMP-LFD exhibits higher sensitivity than LAMP-turbidity, and the LAMP-LFD signal is easily readable to the naked eye (77). Next, using clinical samples, LAMP-LFD and LAMP-turbidity were studied, and the results showed that using LAMP-LFD, the detection of HPV18 and HPV16 had higher sensitivity compared to nested PCR and did not require 2 reaction steps of PCR cycling (77). Finally, LAMP-LFD is a simple and rapid method for highly specific and sensitive HPV detection. Thus, along with the previous HPV18 and HPV16 diagnostic tools, LAMP-LFD might be useful in local hospitals or field studies.