1. Background
Measles is a global occurrence and remains a prominent cause of mortality, particularly among children aged ≤ 5 years. Obtaining precise worldwide incidence estimates is challenging due to heterogeneous surveillance systems and likely under-reporting. The availability of Measles vaccination starting in the 1960s has impacted disease incidence, reducing associated mortality rates, and altering global distribution (1). While the disease primarily affects areas with low vaccination rates, especially in resource-limited settings, Measles outbreaks also emerge in resource-rich environments where vaccination uptake has declined, allowing the transmission of imported measles virus from unvaccinated and infected travelers.
The Measles virus is a single-stranded RNA virus belonging to the Paramyxoviridae family and is classified as a genus of Morbillivirus (1). The virus's antigenically monotypic property enables the production of a live attenuated vaccine from a single strain, demonstrating global favorable efficacy (2). The measles virus is primarily transmitted through respiratory aerosols at short distances and less frequently through particles that remain airborne for an extended period. Epidemics can arise in populations over 15% who lack immunity (3). Transmission chains are common among school-aged children and healthcare workers. Infants become susceptible to the virus when maternal antibodies decrease, and if left unvaccinated, they constitute the most vulnerable cases to the disease.
Rubella is a vaccine-preventable viral illness that typically manifests as a self-limiting condition in children but can have severe effects on the fetus when contracted during pregnancy. It has been described since the 1750s as "German Measles" due to its rash's similarity to Measles and its mention in German literature. The disease is usually a mild febrile illness in childhood, with 25 - 50% of cases being subclinical. However, if the infection occurs early in pregnancy, it can lead to miscarriage, stillbirth, and congenital Rubella syndrome (4). An attenuated live virus Rubella vaccine has been used since the early 1970s to control and eliminate the disease in many industrialized countries. Two primary strategies were considered to halt virus transmission: Selective vaccination of women and universal vaccination of infants (5). Consequently, this not only indirectly protects pregnant women, regardless of vaccination status, but also directly safeguards vaccinated groups against the disease, thereby protecting high-risk populations (6).
Routine immunization with the Measles, Mumps, and Rubella-containing vaccine (MMR) effectively prevents these infections, evident from the decline in cases worldwide following its introduction in the 1960s. However, despite the efforts to eradicate and control Measles and Rubella through immunization for several decades, outbreaks still occur. Thus, assessing the effectiveness of the MMR vaccine has been extensively studied in various populations. The threat to vaccination success comes from international migration and travel to areas where these diseases persist (7).
Due to the global burden of these diseases, various measures have been consistently adopted to control and eliminate them (8). In Iran, vaccination is the pivotal strategy to manage these diseases, while diagnosis (both active and passive) and immediate control measures in high-risk areas contribute to effective management (9). A decrease in maternal antibody levels as children age could render them more susceptible to these diseases (10). Despite antibody waning, the accumulation of susceptible individuals, which increases the potential for new outbreaks, is likely due to the variability in vaccine-induced seropositivity across different populations.
2. Objectives
As a result, this study aims to examine the immunoglobulin G (IgG) antibody levels against these two diseases in infants, preschool children, and pregnant women in Iran.
3. Methods
3.1. Study Population
This descriptive-analytic cross-sectional study was conducted on serum specimens sent to the National Rubella and Measles Reference Laboratory from children with fever and rash under 2 months old, 6, 12, and 18 months old (prior to vaccination), and 5-6-year-old children across the country based on the Ministry of Health Protocol. Additionally, specimens were collected from women at 37 weeks of pregnancy in the admission departments of obstetric wards and hospitals from May to December 2020. The total number of samples was as follows: Children under 2 months (n: 50), 6 (n: 54), 12 (n: 54), and 18 (n: 39) months, and 5-6 years old (n: 49) and women at 37 weeks of pregnancy (n: 53). Exclusion criteria encompassed infection with Acquired Immunodeficiency Syndrome, immunosuppressive drug usage, congenital immunodeficiency diseases (e.g., severe combined immunodeficiency), and deviation from the age-specific vaccination schedule. Specimens exhibiting positive Measles or Rubella IgM were also excluded.
Blood samples from pregnant women were collected in the hospital's midwifery, delivery room, or operating room. For infants and children, samples were obtained from those presenting with fever and rash in accordance with the Ministry of Health protocol, covering all regions of the country (29 out of 31 provinces). Demographic details such as age, gender, underlying diseases, vaccination status, and date of referral were recorded in a designated form. The study age groups encompassed under 2 months (0 - 2 months), 6 months (5 - 7 months), 12 months (prior to the first vaccination), 18 months (prior to the second vaccination), and 6 years old children (5 - 6 years).
3.2. Sampling and Enzyme-Linked Immunosorbent Assay Test
Blood in gel-coated tubes was randomly selected from samples referred to the National Reference Laboratory for Measles and Rubella in Tehran, originating from different provinces of Iran under controlled conditions. Samples unrelated to the defined age groups were excluded from this study. The samples were centrifuged at 3 500 g for 10 minutes to isolate the serums, which were then stored at -80°C until testing. Measuring Measles- and Rubella-specific IgG was conducted using the Mumps and Rubella enzyme-linked immunosorbent assay (ELISA) kit (EUROIMMUN Company, Germany). The results were reported semi-quantitatively by calculating the ratio of sample absorption to calibrator absorption. To detect IgG antibodies in the collected samples, Measles ratio values of < 200 IU/L and ≥ 275 IU/L were regarded as negative and positive, respectively, while Rubella values of < 8 IU/mL and ≥ 11 IU/mL were considered negative and positive. The borderline values within these intervals were designated as equivocal.
3.3. Ethical Considerations
The study protocol underwent review and approval by the Ethics Committee of Shahid Beheshti University of Medical Sciences and Pediatric Infectious Diseases Research Center, Tehran, Iran (IR.SBMU.RICH.REC.1399.060). All results were treated confidentially.
3.4. Statistical Analysis
Categorical data were presented as counts and relative frequencies (percentages). A chi-square test was employed to compare antibody-positive cases among the studied groups. The borderline group was merged with the positive group to prevent sparse data. Antibody concentration was reported as a geometric mean due to the non-normal distribution. Logarithmic values (in base ten) and one-way ANOVA were used to compare study groups. Pairwise comparison has been done by Dunnett's multiple comparison test. All analyses were performed in SPSS (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. Released in 2019), and a P-value of < 0.05 was considered statistically significant.
4. Results
A total of 313 individuals were initially included in this study; however, after excluding cases with incomplete vaccination records, analyses were conducted on 299 cases. Among these 299 cases, 178 (59.53%) were female, including pregnant women. The case distribution in each group was as follows: (1) 50 (16.72%) were infants younger than 2 months; (2) 54 (18.06%) were children aged 6 months; (3) 54 (18.06%) were children aged 12 months; (4) 39 (13.04%) were children aged 18 months; (5) 49 (16.39%) were children aged 5 - 6 years, and 53 (17.73%) were pregnant women. It's noteworthy that samples from the 11 - 13 and 16 - 18 month age groups were taken before vaccination.
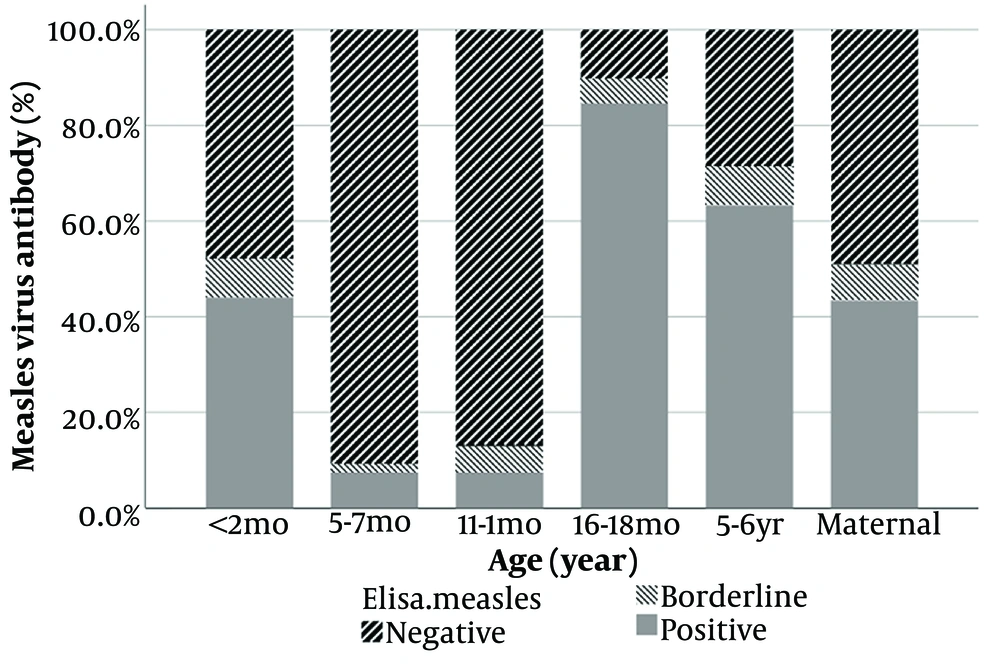
The results revealed significant variation in positive Measles IgG antibody levels among the study groups (P-value < 0.001; see Figure 1). In children, the lowest positive level was observed in the two groups aged 6 and 12 months (7.41%), whereas the highest positive level was found in the 18-month age group (84.62%).
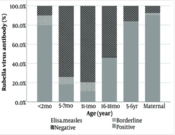
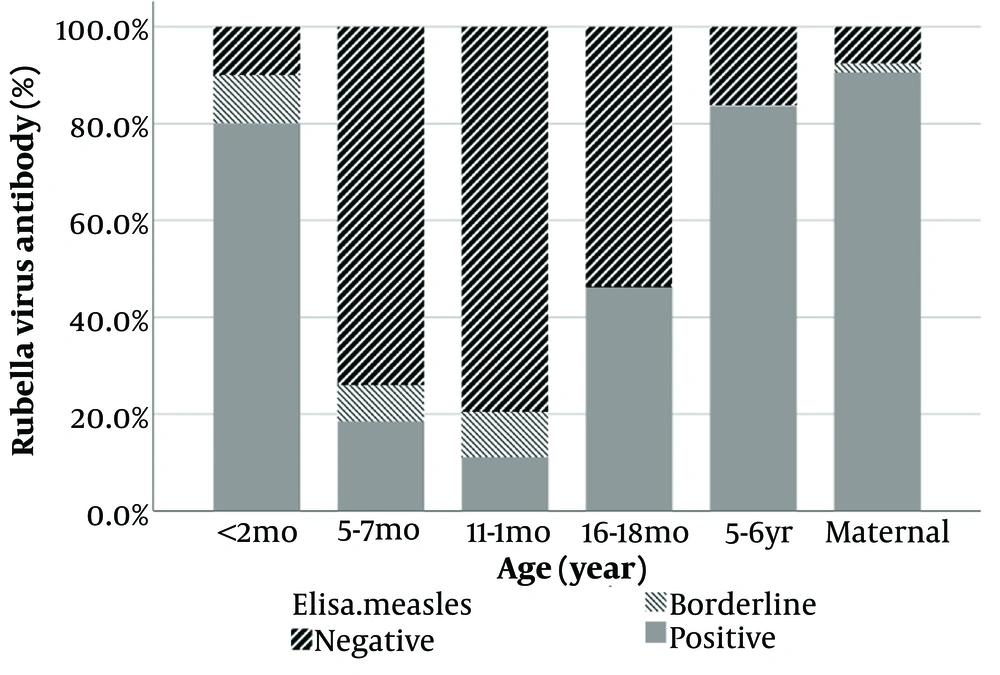
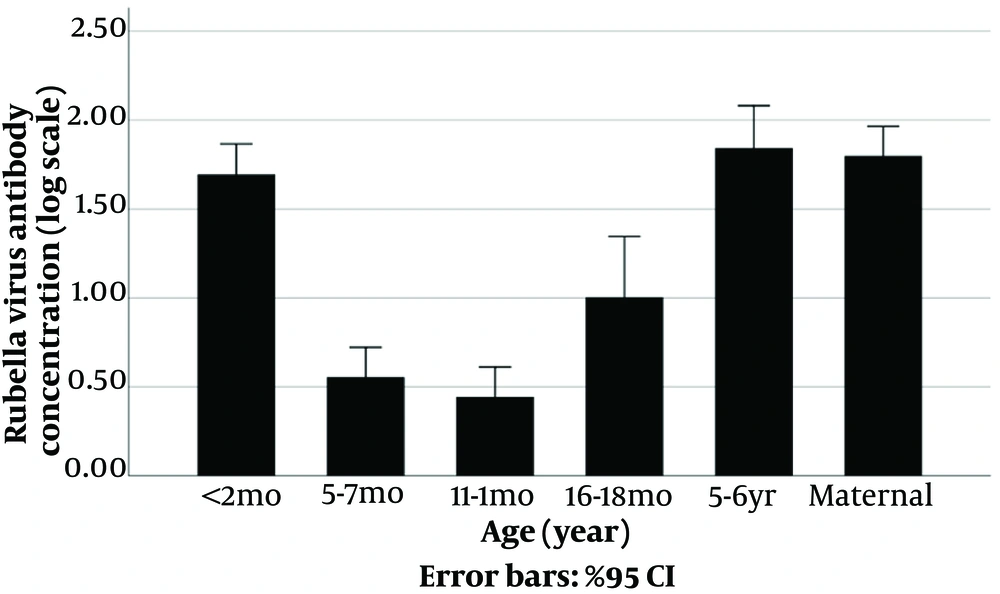
Meanwhile, regarding Rubella IgG, the findings indicated significant differences between study groups (P < 0.001; Figure 2) as well. In children, the least positive level was in 12 months old (11.11%), whereas the most positive level was found in the 5 - 6 years old (83.67%).
Table 1 tabulates the Rubella and Measles IgG antibody concentration measurements in study groups based on geometric means.
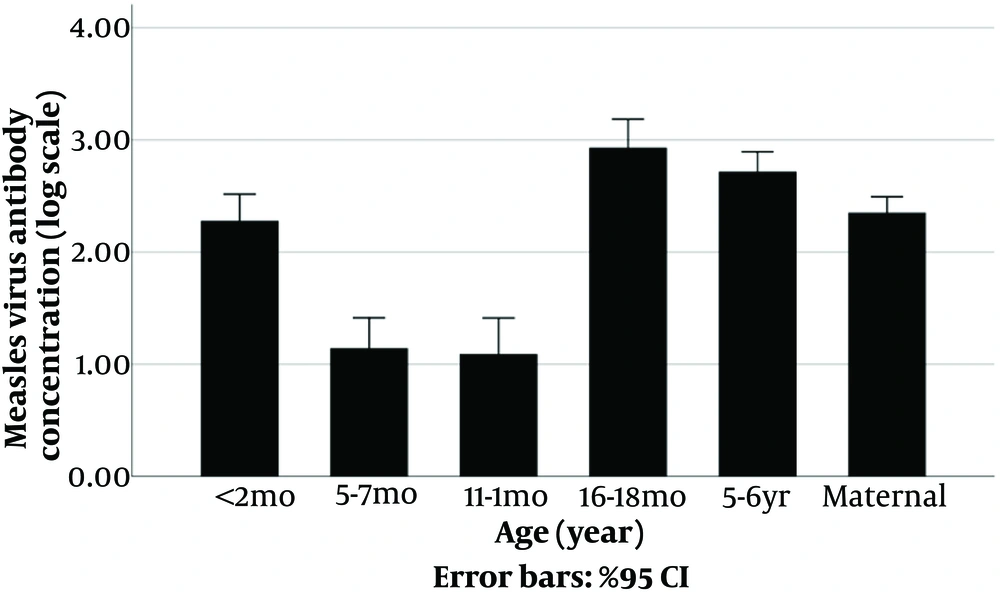
The comparison of the geometric means of Measles and Rubella IgG antibodies between different groups also showed significant differences (P-value < 0.001) (Figures 3 - 4, Appendix 1).
| Age Group | Measles Antibody; Geometric Mean (95% CI) | Rubella Antibody; Geometric Mean (95% CI) |
|---|---|---|
| < 2 months (n = 50) | 190.2 (110.1 to 328.6) | 49.6 (33.5 to 73.5) |
| 6 months (n = 54) | 13.9 (7.5 to 25.9) | 3.6 (2.4 to 5.3) |
| 12 months (n =5 4) | 12.3 (5.9 to 25.8) | 2.8 (1.9 to 4.1) |
| 18 months (n = 39) | 853.7 (476.6 to 1,529.1) | 10.1 (4.6 to 22.2) |
| 5 - 6 years old (n = 49) | 521.5 (346.4 to 785.2) | 69.6 (40.2 to 120.5) |
| Pregnant women (n = 53) | 225.7 (163.2 to 312.2) | 62.9 (42.9 to 92.2) |
Abbreviation: CI, confidence interval.
5. Discussion
Measles and Rubella are recognized as two acute viral diseases primarily affecting children. They contribute significantly to severe morbidity and increased mortality rates among children under 5 years old. The establishment of vaccine-induced immunity plays a pivotal role in preventing these diseases (11).
The World Health Organization (WHO) defines the elimination of Measles as the absence of continuous endemic transmission within a defined geographical area (such as a region or country) for at least 12 months. The World Health Organization also outlines surveillance requirements for Measles and Rubella, including the percentage of clinically identified cases reported within 48 h of symptom onset, the number of rejected cases, the percentage of cases confirmed by virus detection, and the percentage of suspected cases. Specimens should be collected within 48 h of the rash appearance and tested in a WHO-accredited laboratory (12).
World Health Organization-recommended strategies for eliminating Measles and Rubella involve achieving and sustaining over 95% MMR coverage, offering vaccinations to susceptible populations, enhancing surveillance, and improving communication on vaccination benefits and risks (13).
In Iran, adhering to Ministry of Health guidelines, a comprehensive program administered combined MMR vaccines to 95% of individuals aged 2 - 25 years. In conjunction with meeting other WHO criteria, this places Iran on track to achieve Measles and Rubella elimination (14).
Numerous studies conducted across different Iranian provinces and age groups have examined the epidemiology of these diseases (15-17). These studies have unveiled varying susceptibility levels and distinct antibody counts among different age groups and regions, indicating changing patterns over time (18). Regarding immunity in pregnant women, Sharghi et al. executed a systematic review and meta-analysis on Rubella immunity in Iranian women. After reviewing 25 well-conducted studies involving 10 145 women, the pooled prevalence of anti-Rubella IgG-positive cases was 84%. Subgroup analysis indicated a rising IgG prevalence from 78% to 99% between 1989 and 2012 (18, 19). However, our study's Rubella IgG levels among pregnant women (90.57%) differed, possibly due to varied sampling methods and limited regional representation.
A systematic review by Azami et al. reported a 90.1% immunity rate against Rubella and Measles among Iranian pregnant women, linked primarily to age (16). These findings align with our study's Rubella antibody levels. Comparisons of antibody levels among children across countries are challenging due to disparate methods, vaccination schedules, storage conditions, and other factors impacting immunogenicity (20).
For instance, a Canadian study by Guerra et al. revealed that infants were susceptible to Measles and Rubella within the first year, with the focus required on older infants (6 - 12 months) (21, 22). Findings from an American study in 2019 indicated varying protection levels among infants aged 3 - 7 months against Measles and Rubella (23). Studies conducted in China and the Netherlands yielded similar conclusions (24, 25).
Our study indicates lower IgG levels for Measles and Rubella in 5-6-year-olds compared to 16 - 18 months, suggesting potential antibody decline. Furthermore, infants under 12 months old may be most susceptible to both diseases during outbreaks.
5.1. Limitations and Suggestions
In our study, samples were not taken from all across the country, and the distribution of samples was not equal in different provinces.
5.2. Conclusions
In conclusion, it can be stated that Measles and Rubella antibody titers were lower in children 12 months before vaccination and reached a positive level in children aged 18 months post-vaccination. Meanwhile, Measles IgG levels were lower in 5-6-year-old children compared to 18-month-old children. Meanwhile, pregnant women exhibited high levels of protection against these viruses (more than 80% had positive Rubella IgG).
The findings indicated that the antibody levels among Iranian children and pregnant women aligned with the anticipated patterns.