1. Background
Diarrhea remains a significant contributor to high morbidity and mortality among children, especially in developing nations. Among children under 5 years old, acute diarrhea ranks as the fifth most prevalent ailment, with approximately 1.7 billion cases annually, resulting in 446 000 child deaths each year (1). The origins of childhood diarrhea can be attributed to infectious or non-infectious causes. Notably, Rotavirus infection emerges as the foremost and consistent factor behind acute diarrhea, particularly in low- and middle-income countries (2). In Vietnam, Rotavirus infection holds paramount importance as a causative agent of acute diarrhea in hospitalized children. Its prevalence, as identified through various studies, exhibits significant variation yet consistently accounts for a substantial proportion, ranging from 46.7% to 68.8% (3-5). The phenomenon of co-infection is prevalent and spans a wide spectrum of conditions, from common respiratory ailments like severe community-acquired pneumonia to gastrointestinal infections such as Rotavirus-induced acute diarrhea (6-8). The occurrence of Rotavirus co-infection has been reported within a range of 22% to 60% (9, 10).
The clinical manifestations of Rotavirus infection encompass a broad range, extending from asymptomatic cases to severe and life-threatening conditions marked by symptoms such as diarrhea, vomiting, fever, and dehydration. In comparison to other causative agents, Rotavirus infection tends to induce more pronounced watery diarrhea, vomiting, and fevers in children (11, 12). Consequently, the significance of co-infections involving microbial agents and Rotavirus has garnered heightened attention, prompting a deeper exploration into the role of co-infections. Traditional approaches to pathogen identification often involve bacterial isolation and cultivation. However, these methods are not always capable of identifying the root cause of the disease, and the time required for determination through conventional culture techniques can impede timely treatment. Real-time polymerase chain reaction (PCR) has emerged as a highly sensitive and specific technology for early detection of infectious agents, serving as a vital tool in clinical practice (13). Therefore, this technique was applied in this study to detect microbial agents in children with acute diarrhea.
2. Objectives
This study aimed to determine the frequency distribution of Rotavirus infection and relevant co-infections in children with acute diarrhea; and to compare the analysis of clinical characteristics and laboratory findings between 2 distinct groups: Rotavirus infected and Rotavirus uninfected group; Rotavirus mono-infection and co-infection group.
3. Methods
3.1. Study Design and Data Collection
A cross-sectional study was conducted on 171 children at the Department of Gastroenterology in Can Tho Children′s Hospital, located in Can Tho City, Vietnam, from November 2022 to April 2023.
The inclusion criteria were children aged 2 to 5 years old who had been diagnosed with acute diarrhea and had been hospitalized within 48 hours. Acute diarrhea was defined as the passage of abnormally loose or watery stools 3 or more times in 24 hours, lasting no longer than 14 days (1).
The exclusion criteria included children with gastrointestinal disorders that cause diarrhea, malabsorption, chronic diseases, or immune disorders.
The sample size was calculated using the following formula:
Where “n” is the minimum sample size; “α” represents the probability of a type 1 error (α = 0.05); “Z” denotes the value of the standardized normal distribution (Z = 1.96); “d” represents the allowable error (d = 0.08); “p” corresponds to the rate of detecting the agent causing acute diarrhea caused by Rotavirus, as reported by Huyen et al. in 4 sentinel surveillance hospitals in Vietnam during the period 2012 - 2015 (P = 0.467) (3).
Therefore, the minimum required sample size “n” was calculated to be 150. However, the study ultimately included 171 patients. The sampling method was convenient until a sufficient number of samples was obtained.
Children who met the inclusion criteria and were hospitalized were invited to participate in this study. All patients underwent clinical examinations, including assessments of fever, vomiting, watery stool, the highest number of loose stools per day, and dehydration levels. Blood counts were conducted using the Siemens ADVIA® 2120i hematology analyzer (Siemens Healthineers, Erlangen, Germany). Serum sodium, serum potassium, and C-reactive protein (CRP) levels were measured using the AU480 biochemical analyzer (Beckman Coulter, Brea, California, USA).
Rectal swabs were obtained from all patients using a Nam Khoa Swab Specimen Collection Kit (Nam Khoa Company, Vietnam). The swab was carefully inserted into the child′s rectum, 1 - 2 inches (3 - 5 cm) past the anal margin or the outside of the anus, by the collector. The swab was then gently rotated clockwise for 5 to 10 seconds. Subsequently, the swab was removed without contacting the patient′s skin and placed into the transfer tube immediately. After securely capping the tube, it was delivered within 24 hours via the Premium Delivery Service of Nhat Tin Logistics to the International Institute of Genetics and Immunology at Nam Khoa Services and Trading Company Limited in Ho Chi Minh City, Vietnam, for real-time PCR analysis. This laboratory adheres to Vietnamese standards ISO 9001:2015 and 13485:2017, as well as WHO-GMP (TRS 908, ANNEX 4).
The process of implementing the real-time PCR can be summarized as follows:
To homogenize the sample, we combined 3 mL of the specimen with 10 mL of clean water containing 50 mg of N-acetyl L-cysteine (NALC). The mixture was centrifuged in a benchtop centrifuge at the highest speed for 15 minutes; then, the residue (approximately 300 µL) was removed for DNA extraction.
Nucleic acid extraction was performed using a ZiXpress-32® machine (Zinexts Life Science Corp, Taiwan) with the NKDNARNAprep-MAGBEAD reagent kit (Nam Khoa Company, Vietnam), which was validated through comparison with the BOOM method for nucleic acid extraction.
Nucleic acid extracts from patient samples underwent real-time PCR using the CFX96 TouchTM system (Bio-Rad Laboratories, USA). This involved the use of specific primers and TaqMan probes designed to detect the 42 microbial agents responsible for acute diarrhea in children (see Appendix 1). Viral and bacterial coinfection was defined as the presence of both viruses and bacteria in a specimen.
3.2. Statistical Analysis
Quantitative variables were presented as medians along with interquartile ranges (in cases of non-normal distribution), while qualitative variables were expressed as percentages. The Mann-Whitney U test was used to compare quantitative variables without a normal distribution. For categorical variables, the χ2 or Fisher′s exact test was applied. The analysis was performed using SPSS v. 22.0 (IBM Corp., Armonk, NY, USA), and a significance level of P < 0.05 was considered.
3.3. Ethical Approval
This study received ethical approval from the Ethics Committee in Biomedical Research of Can Tho University of Medicine and Pharmacy under reference number 22.149.SV/PCT-HDĐD.
4. Results
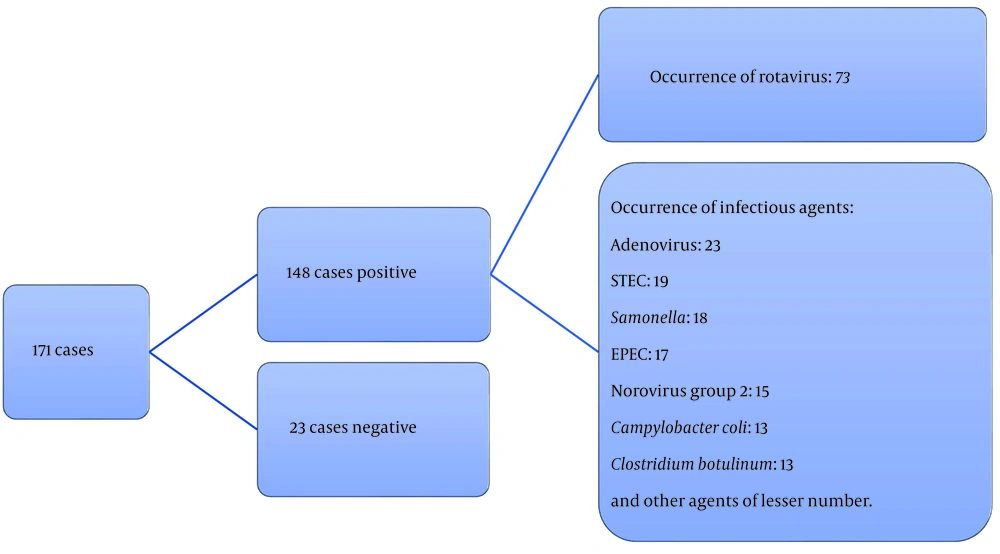
Between November 2022 and April 2023, a total of 171 children participated in the study. Upon analyzing rectal swab samples from these participants, the research team observed that Rotavirus was the predominant pathogen, accounting for 73 out of 171 cases (42.7%), as illustrated in Figure 1.
As detailed in Table 1, co-infections were identified in 45.2% of children within the Rotavirus-positive group (33 out of 73). Among these, 28.8% (21 out of 73) exhibited co-infections with a single agent, while 16.4% (12 out of 73) had co-infections involving 2 or more agents. The most common co-infection was observed between Rotavirus and enteropathogenic Escherichia coli (EPEC), with a prevalence of 24.2% (8 out of 33). This was followed by co-infections between Rotavirus and Campylobacter coli, accounting for 21.2% (7 out of 33). Co-infections involving other agents, while less frequent, included Shiga toxin-producing E. coli (STEC), Shigella sp., Campylobacter jejuni, Clostridium difficile, E. coli O157:H7, enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), norovirus group II, aichivirus, bocavirus, adenovirus, Dientamoeba fragilis, and Balantidium coli (Table 1).
| Rotavirus Infection Results | Viral and Bacterial Co-infections | Frequency; n | Total; No. (%) |
|---|---|---|---|
| Co-infection with more than 2 agents | Rotavirus, EPEC, STEC, ETEC, Shigella. | 1 | 12 (16.4) |
| Rotavirus, EPEC, Shigella sp., Campylobacter coli | 1 | ||
| Rotavirus, EPEC, STEC | 3 | ||
| Rotavirus, EPEC, ETEC | 2 | ||
| Rotavirus, EPEC, Campylobacter coli | 1 | ||
| Rotavirus, Shigella sp., Campylobacter jejuni | 1 | ||
| Rotavirus, Campylobacter coli, Campylobacter jejuni | 1 | ||
| Rotavirus, Salmonella, adenovirus | 1 | ||
| Rotavirus, norovirus, adenovirus | 1 | ||
| Co-infection with 1 agent | Rotavirus, Campylobacter jejuni | 5 | 21 (28.8) |
| Rotavirus, Dientamoeba fragilis | 3 | ||
| Rotavirus, Clostridium difficile | 2 | ||
| Rotavirus, Shigella sp. | 3 | ||
| Rotavirus, Bocavirus | 1 | ||
| Rotavirus, Campylobacter coli | 1 | ||
| Rotavirus, EIEC | 1 | ||
| Rotavirus, norovirus | 1 | ||
| Rotavirus, adenovirus | 1 | ||
| Rotavirus, Salmonella | 1 | ||
| Rotavirus, Vibrio parahaemolyticus | 1 | ||
| Rotavirus, Balantidium coli | 1 | ||
| Mono-infection | Rotavirus | 40 | 40 (54.8) |
| Total | 73 | 73 (100) |
Viral and Bacterial Co-infections in Children with Rotavirus Acute Diarrhea (n = 73)
Compared to the Rotavirus-negative group, a higher proportion of children in the Rotavirus-positive group exhibited watery stools (P = 0.004), vomiting (P < 0.001), and a higher frequency of loose stools per day (P = 0.007). However, there were no notable differences between the groups in terms of fever, dehydration levels, hematocrit value, hemoglobin value, white blood cell count, serum sodium, serum potassium, and CRP value (all P > 0.05), as depicted in Table 2.
| Characteristics | Rotavirus-Infected Children (n = 73) | Rotavirus Un-infected Children (n = 98) | P-Value |
|---|---|---|---|
| Clinical features | |||
| Fever | 63 (86.3) | 77 (78.6) | 0.194 |
| Vomiting | 65 (89.0) | 56 (57.1) | < 0.001 b |
| Watery stool | 43 (58.9) | 36 (36.7) | 0.004 b |
| The greatest number of diarrhea/days | 7 (5 - 10) | 5.5 (4 - 8) | 0.007 b |
| Dehydration levels | 0.116 | ||
| Severe dehydration | 0 | 0 | |
| Some dehydration | 16 (21.9) | 12 (12.8) | |
| No dehydration | 57 (78.1) | 86 (87.2) | |
| Laboratory data | |||
| Hemoglobin (g/L) | 115.5 (108.0 - 125.0) | 112 (103 - 120.25) | 0.056 |
| Hematocrit (L/L) | 0.362 (0.330 - 0.386) | 0.343 (0.319 -0.370) | 0.647 |
| White cell count (109 cell/L) | 9.6 (7.11 - 12.75) | 10.4 (8.2 - 14.0) | 0.127 |
| Serum sodium (mmol/L) c | 134.25 (130.85 - 138.33) | 133.2 (130.4 - 139.9) | 0.743 |
| Serum potassium (mmol/L) c | 3.64 (3.155 - 4.063) | 3.90 (3.20 - 4.23) | 0.406 |
| CRP (mg/L) c | 10.2 (7.95 - 17) | 8.7 (6.87 - 18.73) | 0.507 |
Characteristics of Rotavirus Infected and Rotavirus Uninfected Children a
When comparing characteristics between the 2 groups-Rotavirus mono-infection and co-infection-in children with acute Rotavirus diarrhea, it was observed that children in the Rotavirus mono-infected group experienced more instances of vomiting compared to those in the co-infected group (P = 0.019). Notably, no significant distinctions were found in terms of watery stools, fever, frequency of loose stools, hemoglobin value, hematocrit index value, white blood cell count, serum sodium, serum potassium, CRP value, and dehydration levels between the 2 groups (P > 0.05), as displayed in Table 3.
| Characteristics | Rotavirus Mono-infection Group (n = 40) | Rotavirus Co-infection Group (n = 33) | P-Value |
|---|---|---|---|
| Clinical features | |||
| Fever | 34 (85) | 29 (87.9) | 1.000 |
| Vomiting | 39 (97.5) | 26 (78.8) | 0.019 b |
| Watery stool | 21 (52.5) | 22 (66.7) | 0.221 |
| The most number of diarrhea/day | 7 (5-10) | 7 (4-10) | 0.978 |
| Dehydration levels | 0.895 | ||
| Severe dehydration | 0 | 0 | |
| Some dehydration | 9 (22.5) | 7 (21.2) | |
| No dehydration | 31 (77.5) | 26 (78.8) | |
| Laboratory data | |||
| Hemoglobin (g/L) | 117 (109 - 128) | 114 (107.25 - 123.75) | 0.359 |
| Hematocrit (L/L) | 0.358 (0.332 - 0.391) | 0.362 (0.329 - 0.383) | 0.471 |
| White cell count (109 cell/L) | 10.9 (7.2 - 14.2) | 9.0 (7.0 - 11.4) | 0.066 b |
| Serum sodium (mmol/L) c | 134.25 (131.45 - 139.35) | 134.25 (130.53 - 136.95) | 0.601 |
| Serum potassium (mmol/L) c | 3.66 (3.19 - 4.21) | 3.63 (2.76 - 3.74) | 0.272 |
| CRP (mg/L) c | 11 (8.08 - 16.5) | 10 (7 - 26.8) | 0.681 |
Characteristics of Rotavirus Mono-infection and Microbial Co-infection in Children a
5. Discussion
Similar findings have been reported in numerous domestic and international research studies regarding the rate of Rotavirus infection. For instance, Zhang et al. reported that 42% of children in China experienced Rotavirus-induced diarrhea based on data from the Chinese Health Statistic Yearbook, the Viral Diarrhea Surveillance System in China, and peer-reviewed publications spanning from 2003 to 2012 (14). When Jain et al. employed real-time PCR technology to test for microbiological agents in patients with diarrhea in India, they found that up to 49.5% of cases tested positive for Rotavirus, which was the most frequently identified agent (15). A comprehensive study was conducted in Vietnam between 2012 and 2015 at 4 hospitals - National Children′s Hospital (Hanoi), Children′s Hospital 1 (Ho Chi Minh City), Khanh Hoa General Hospital, and Ninh Hoa District General Hospital (Khanh Hoa City) - involving 8 889 children under the age of 5, which revealed that Rotavirus was the most common pathogen, accounting for 46.7% of cases (3).
However, our identified incidence was lower than that reported by Doan et al. in Ho Chi Minh City, Vietnam (65.6%), and Van Chuc et al. in Hai Phong City, Vietnam (68.8%) (4, 5). Our study showed a higher risk of Rotavirus infection compared to other research studies. Chen et al.′s research at Chang Gung Children′s Hospital in Taiwan, which used real-time PCR to detect microbiological agents causing acute diarrhea from August 2004 to January 2007, reported a Rotavirus infection rate of 28.6% (16). Ojobor et al. conducted an epidemiological investigation in Nigeria to ascertain the Rotavirus prevalence in Enugu state, and they discovered that 31.5% of children with acute diarrhea had Rotavirus infection (17).
According to the medical literature, Rotavirus-induced diarrhea contributes to 50% to 65% of all cases of acute diarrhea in hospitalized children. Regional and international variations in Rotavirus infection rates can be attributed to factors such as climatic conditions, the environment, socioeconomic factors, testing methodologies, studied populations, and the duration of the studies.
The rate of microbial co-infection among children infected with Rotavirus was 45.2% (33 out of 73). In comparison to several other studies, this co-infection rate is lower. Between May 2015 and April 2016, Ghapoutsa et al. conducted a research on hospitalized children under 5 years old with acute gastroenteritis in hospitals in the Cameroonian Littoral region. They discovered that 34 out of 54 patients, or 60% of cases, had co-infections with Rotavirus and other microbes (7). In research conducted between June 2015 and April 2016 in India by Shrivastava et al., out of 34 instances of Rotavirus infection, 19 cases had co-infections, yielding a prevalence of 55.9% (10). Our co-infection rate, however, is higher than that of a study conducted in the UK between June 2011 and December 2013 by Karampatsas et al. In that investigation, pathogen presence was verified by PCR, and a Rotavirus coinfection rate of 22% (11 out of 50) was obtained (9). Distinctions in socioeconomic status and geographical location seemingly influence the emergence of co-infectious agents. The most common co-infections detected alongside Rotavirus were EPEC. In a study involving 130 children under 5 years of age with 3 or more diarrheal episodes in a day in a tertiary care teaching hospital in Bhubaneswar, Odisha (India), it was found that Shigella sp. was the most frequent co-infectious agent (52.6%), closely followed by EPEC (47.4%) (10). Moreover, EPEC ranked second to enteraggregative E. coli (EAEC) in terms of microbial co-infections with Rotavirus, as identified by Nguyen et al. in Ha Noi, Vietnam (18). These observations underscore the high prevalence of co-infections, particularly involving bacterial agents, alongside Rotavirus.
Our analysis demonstrated that children with Rotavirus-positive cases exhibited a higher prevalence of watery stools, vomiting, and a higher frequency of loose stools per day compared to Rotavirus-negative cases (P < 0.05). These findings align with well-documented evidence in medical literature and prior studies, as Rotavirus-induced gastrointestinal disease typically presents with watery stools, mild fever, vomiting, and mild inflammation causing intestinal damage (12, 19, 20). It is worth noting that most children affected by acute Rotavirus diarrhea experienced fever (86.3%); however, this difference was not statistically significant between children with and without Rotavirus infection. Notably, studies by Karampatsas et al. and Ojobor et al. both reported higher instances of fever in children with Rotavirus infection compared to those without (9, 17). The presence of other pathogens capable of causing fever (such as norovirus, adenovirus, astrovirus, E. coli, and Salmonella spp.) in the Rotavirus-negative group could potentially contribute to an increased fever rate (78.6%) within this cohort. The rate of dehydration (21.9%) in Rotavirus-positive cases was lower than findings from domestic and foreign studies, which reported rates ranging from 30% to 47% (5, 21). Nonetheless, no significant difference was observed in the dehydration levels between the 2 groups. The study failed to identify statistically significant differences in hematocrit value, hemoglobin value, white blood cell count, serum sodium, serum potassium, or CRP value between Rotavirus-infected and non-infected groups. Interestingly, this aligns with findings by Karampatsas et al. in the UK in 2018, where they similarly found no significant disparities in blood sodium, blood potassium, or CRP value between the 2 groups (9).
When comparing clinical features and degrees of dehydration between the Rotavirus mono-infection and co-infection groups, no significant differences were identified in watery stools, fever, the frequency of diarrhea, and dehydration levels between the 2 groups. These findings align with the outcomes of several other research studies. From March 2001 to April 2022, Nguyen et al. conducted research in Hanoi on Rotavirus and bacterial agents that induce diarrhea in 587 children. The authors observed that concurrent Rotavirus and bacterial infections did not significantly worsen the disease compared to mono-infection when comparing clinical features such as fever, vomiting, and dehydration between the groups infected with Rotavirus and the group infected with a combination of Rotavirus and bacteria (18). Similar findings were reported by Koh et al. in their research on viral co-infections in Korean children, where there was no difference in fever, vomiting, or the duration of diarrhea between acute diarrhea caused by viral agent coinfection and acute diarrhea caused by mono-infection with viral agents (22).
Additionally, in an investigation carried out between February 2008 and June 2010 by Matthijnssens et al., stool samples from Rotavirus-related gastroenteritis cases in 39 Belgian hospitals were collected to assess the impact of co-infected pathogens on disease severity. The outcomes demonstrated that there was no discernible clinical difference between cases of co-infection with different viral agents and mono-infection with Rotavirus (8). Moreover, Moyo et al. (23) in Tanzania in 2017 discovered that no significant differences existed. We did not detect differences in electrolytes and CRP between the Rotavirus mono-infection and co-infection groups. This further confirms that clinical and paraclinical characteristics are compatible with each other.
The study′s advantage lies in the use of real-time PCR, a highly sensitive and specific method that accurately detects pathogens. However, the study has limitations as nutritional status, vaccination history, and previous diarrhea history were not recorded to make comparisons between the Rotavirus-infected and Rotavirus-uninfected groups, as well as between the Rotavirus mono-infected and co-infected groups. These factors could potentially influence the severity of diarrhea in this disease.
In Vietnam, a significant percentage of children experienced acute diarrhea caused by Rotavirus and microbial co-infection with Rotavirus. However, co-infections did not have an impact on the clinical characteristics or laboratory data related to acute diarrhea. Therefore, it is more crucial to identify Rotavirus in children experiencing acute diarrhea than to identify any co-infections.