1. Background
Rituximab (RTX) is a chimeric monoclonal antibody of human-murine origin (IgG1 kappa, with human Fc) that specifically targets CD20 receptors on both normal and malignant B-lymphocytes, subsequently leading to the depletion of these cells through apoptosis induction (1, 2). RTX is extensively used in the treatment of hematological malignancies and autoimmune diseases (3-6). The pharmacokinetics of RTX vary between patients with malignancies and those with hematologic disorders, influenced by factors such as tumor burden; higher tumor burdens are associated with lower RTX levels. Studies have documented varied pharmacokinetic profiles of RTX across different diseases, highlighting that its clearance is affected by the number of target molecules and the tumor burden. The recommended RTX dosage is higher for patients with chronic lymphocytic leukemia (CLL) than for those with non-Hodgkin lymphoma (NHL), underscoring that antigen burden significantly influences RTX exposure. The pharmacokinetics and pharmacodynamics of RTX are intricate, subject to alterations by numerous factors including the type of disease, treatment regimen, and antigen burden (7-9).
RTX has been linked to significant side effects, with immunosuppression being one of the most critical (10, 11). While typically transient, immunosuppression may persist for up to 12 months. RTX increases the risk of bacterial, viral, and fungal infections (12-15). Notably, RTX has been associated with more severe infectious outcomes in adults than in children. Research on RTX's infectious side effects in pediatric patients, particularly those with non-malignant disorders, is limited, with most of the available data derived from adult studies (16, 17).
Current practices for utilizing RTX draw upon our understanding of how biologic response modifiers (BRMs) affect the immune system. Yet, the implications of concurrently using BRMs with other immunosuppressive medications remain less clear.
2. Objectives
This cohort study was designed to explore RTX-related infectious complications and survival rates among children diagnosed with malignancy and chronic immune thrombocytopenic purpura (ITP). We monitored RTX-treated patients for the development of febrile neutropenia (FN), bacterial bloodstream infections (BSI), invasive fungal infections (IFI), and mortality during an extended period of observation.
3. Methods
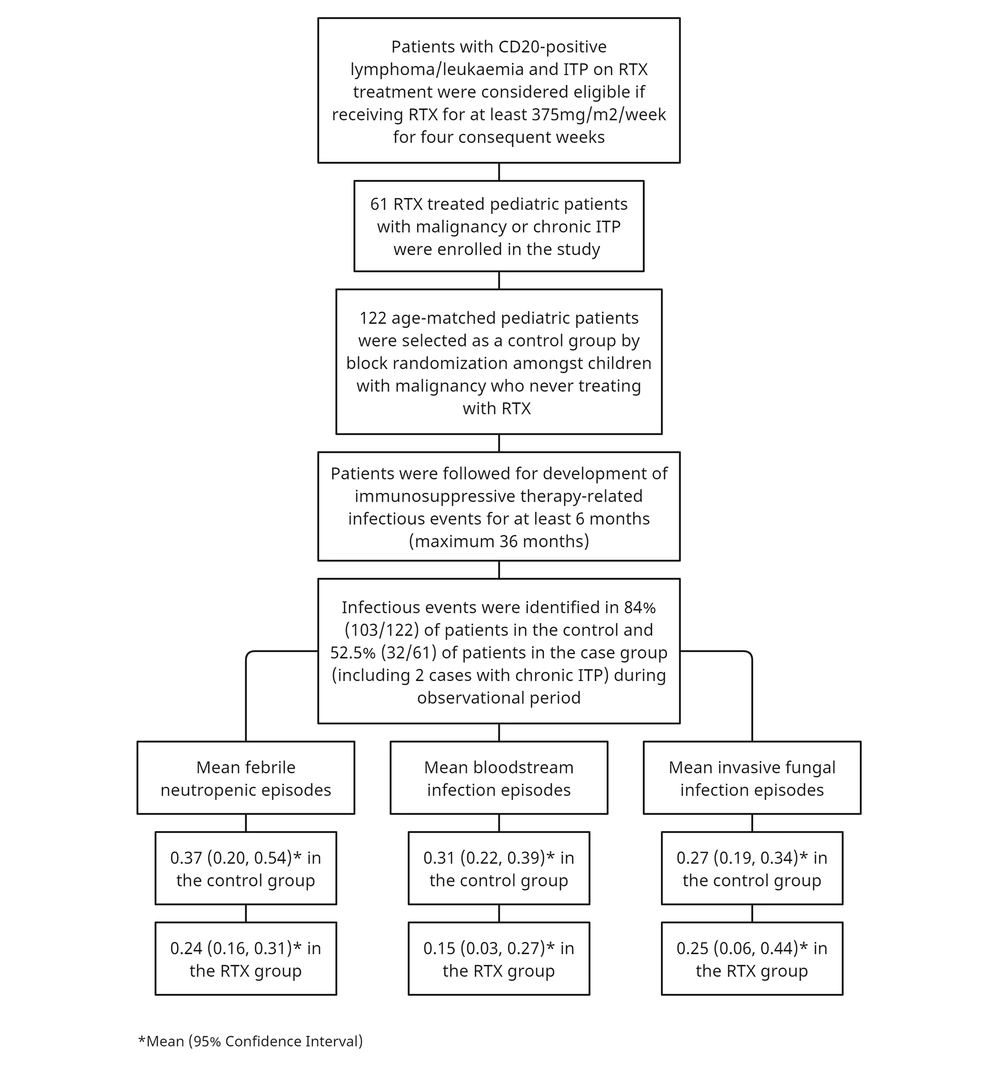
In this observational cohort study, pediatric patients undergoing RTX treatment were monitored for the occurrence of predefined endpoints at the Amir Medical Oncology Center over a 36-month follow-up period (for the definition of terms, see the appendix file). The study included patients with CD20-positive Hodgkin/non-Hodgkin lymphomas and chronic immune thrombocytopenic purpura (ITP) who were receiving RTX treatment as the exposed group. Eligibility required receiving RTX at a dose of at least 375mg/m2/week for four consecutive weeks. An unexposed group was identified through block randomization among children with malignancies who had never received RTX. The majority of cases in the unexposed group were children diagnosed with acute lymphoblastic leukemia (ALL) and acute myeloblastic leukemia (AML). Sixty-one patients receiving RTX therapy were enrolled, and the unexposed group consisted of 122 age-matched pediatric cancer patients who had never been treated with RTX (for additional information, refer to the study flowchart (Figure 1)).
3.1. Data analysis
Data were coded, entered, and analyzed using SPSS version 21.0 (SPSS Software, CA, USA). The Mann-Whitney U test was applied to non-normally distributed parametric data, while the Student's t-test was utilized for normally distributed numerical data, with results reported as mean ± SD. Both the Chi-square and Fisher's exact tests were employed to evaluate categorical variables, presented as frequency and percentage. Logistic regression analysis, employing a backward elimination method, was conducted to identify predictors of outcome variables using Stata software (Version 17; StataCorp, Texas, USA). To assess multicollinearity, the variation inflation factor (VIF) was calculated. An adjusted odds ratio (OR) with a 95% confidence interval (CI) and a two-sided P-value < 0.05 were employed to determine the strength of associations. All independent variables with a P-value < 0.2 in univariate analysis were considered for inclusion in the multivariable logistic regression. The mean durations of all infectious events and mortality were analyzed using the Kaplan-Meier test, with the survival time differences between the exposed and unexposed groups examined through the Log Rank (Mantel-Cox) test. Hazard risk, defined as the probability of death or the occurrence of predefined endpoints (under the proportional hazards assumption), provided the patient survived until the last visit. The cumulative proportional survival rate by the end of the observation period was estimated using the survival life table.
All procedures carried out in this study involving human participants adhered to the ethical standards of the institutional and/or national research committee, in accordance with the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical norms.
4. Results
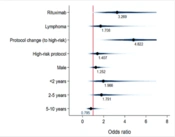
The study included 183 patients with malignancies, chronic immune thrombocytopenic purpura (ITP), or hematologic disorders. Of these, 61 were treated with RTX and formed the exposed group, while 122 constituted the unexposed group. The average age (± SD) was 7.83 ± 4.56 years for the exposed group and 7.13 ± 5.09 years for the unexposed group (P-value: 0.209). Cancer patients represented 68.9% (42/61) of the exposed group and 95.1% (116/122) of the unexposed group, respectively (Appendix 1). The study also included 19 ITP cases treated with RTX, which were evaluated for infectious events and mortality during the observation period. Among the children with malignancies, 76.2% had CD20+ lymphomas, and there were four cases of CD20+ acute lymphoblastic leukemia (ALL).
Infectious complications were reported in 52.5% (32/61) of the children treated with RTX, whereas 84.4% (103/122) of the patients in the unexposed group experienced infections. The rate of infectious complications was notably higher in children with malignancy than in those with chronic ITP (89.5% versus 10.5%, respectively). Among the chronic ITP cases, only two developed FN and BSI (Appendix 1).
Demographic and clinical characteristics of 158 patients with malignancies (excluding those with chronic immune thrombocytopenic purpura or hematologic disorders) are presented in Table 1. According to the EORTC/MSG criteria (18), proven, probable, and possible IFIs were identified in 5, 9, and 10 cases, respectively. Cases of candidemia, invasive pulmonary aspergillosis, and mucormycosis were reported in 10, 10, and 4 patients, respectively.
| Variables | Exposed (42) | Unexposed (116) | P-Value |
|---|---|---|---|
| Gender | |||
| Male | 32 (76.2) | 64 (55.2) | 0.017 b |
| Female | 10 (23.8) | 52 (44.8) | |
| Age, year | |||
| < 2 | 1 (2.4) | 14 (12.1) | 0.104 |
| 2 - 5 | 7 (16.7) | 31 (26.7) | |
| 5 - 10 | 18 (42.9) | 38 (32.8) | |
| > 10 | 16 (38.1) | 33 (28.4) | |
| Underlying disease | |||
| Leukemia | 4 (9.5) | 56 (48.3) | < 0.001 b |
| Lymphoma | 32 (76.2) | 6 (5.2) | |
| Other malignancies | 6 (14.3) | 54 (46.6) | |
| Baseline protocol risk | |||
| Standard risk | 9 (21.4) | 72 (62.1) | < 0.001 b |
| High risk | 33 (78.6) | 44 (37.9) | |
| Change to high-risk protocol | 8 (19.0) | 33 (28.4) | 0.234 |
| RTX dose, mg | 1273.81 ± 997.81 | - | - |
| Infectious events | 30 (71.4) | 102 (87.9) | 0.013 b |
| FN | 29 (69.0) | 94 (81.0) | 0.109 |
| BSI | 6 (14.3) | 34 (29.3) | 0.055 |
| IFI | 6 (14.3) | 18 (15.5) | 0.849 |
| All-cause mortality | 12 (28.6) | 30 (25.9) | 0.733 |
Abbreviations: ITP, immune thrombocytopenic purpura; RTX, rituximab; FN, febrile neutropenia; BSI, bloodstream infection; IFI, invasive fungal infection.
a Values are expressed as mean ± SD or No. (%).
b Chi-square test.
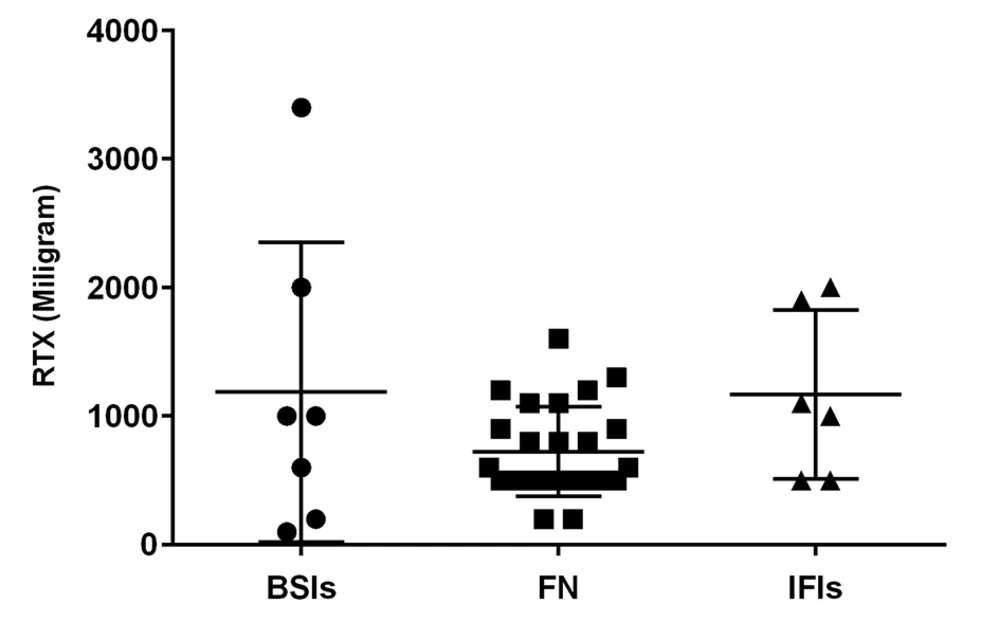
The mean total dose of RTX administered prior to the first infectious events (BSI, FN, and IFI) was calculated, revealing no statistically significant differences (Figure 2). Due to the small number of infectious episodes in patients with chronic ITP (2 cases), logistic regression and survival analysis were conducted on children with malignancy to ensure an appropriate number of events per variable.
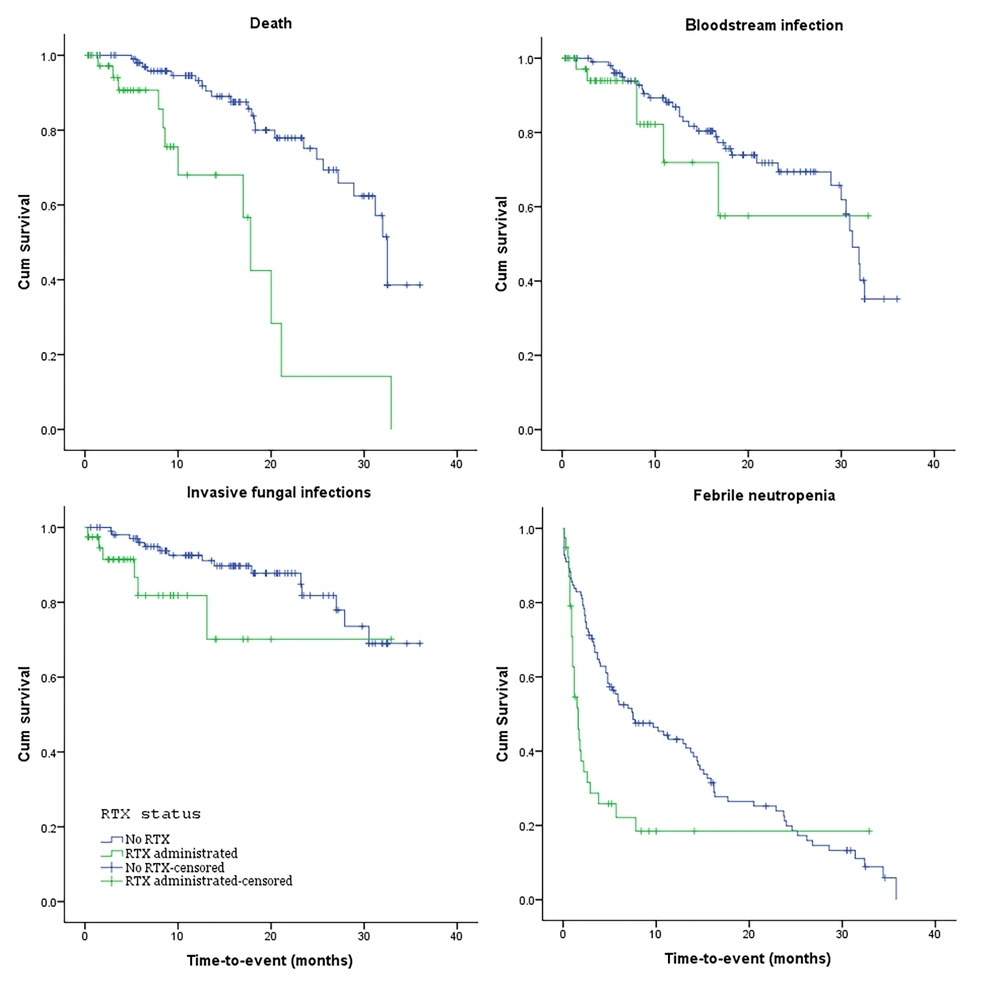
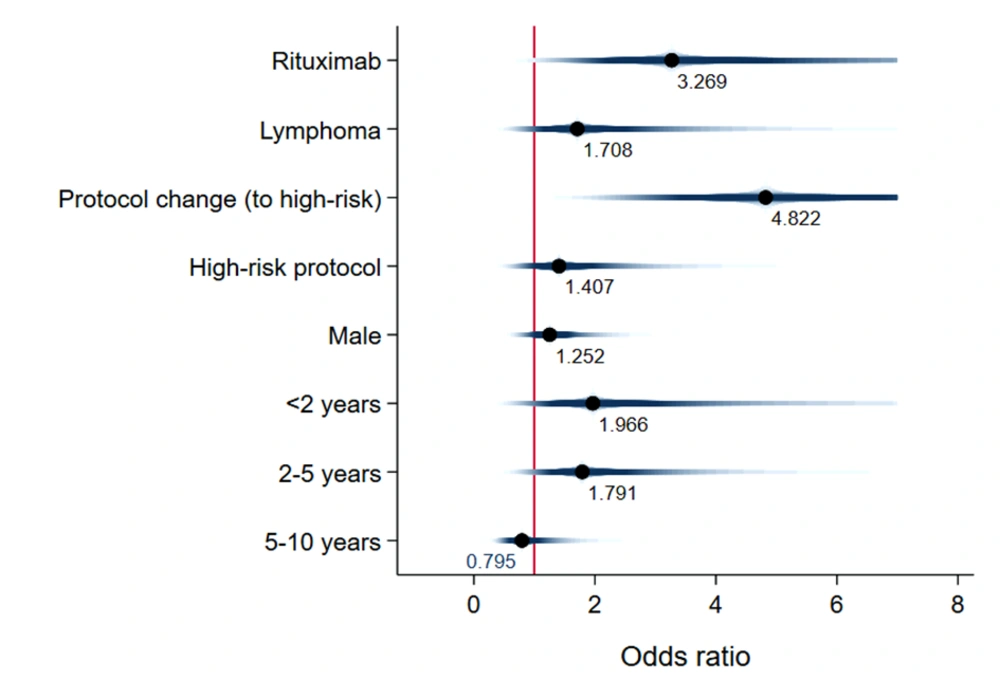
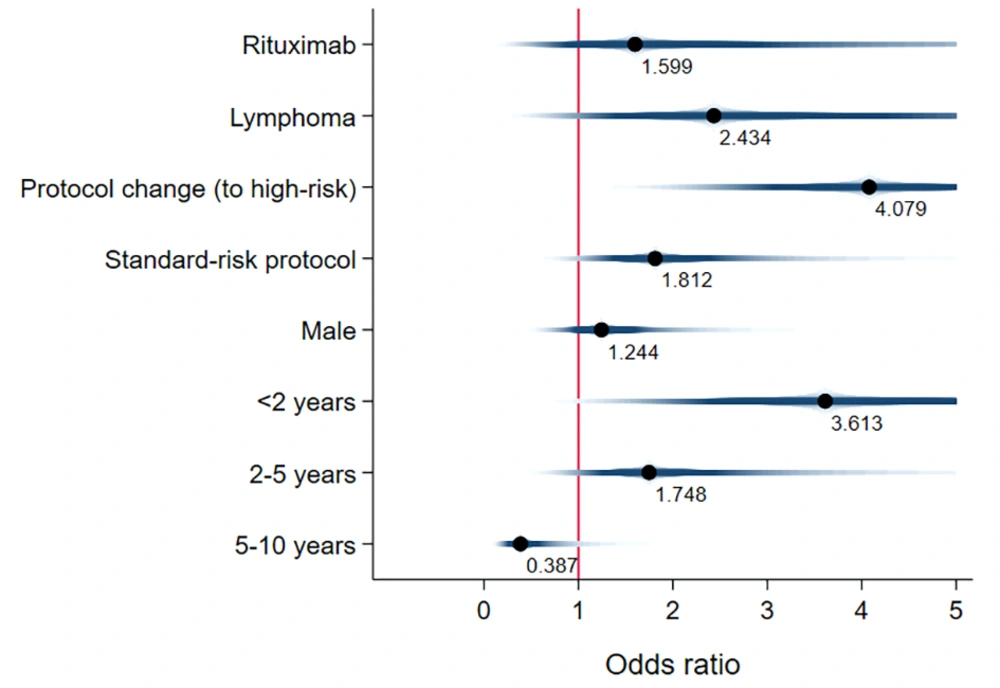
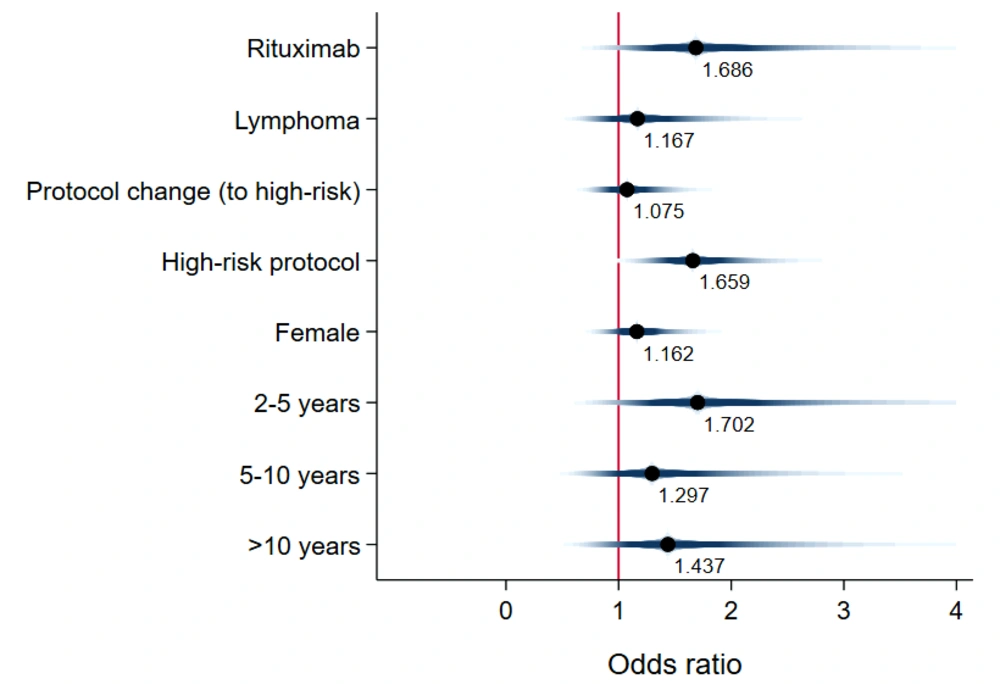
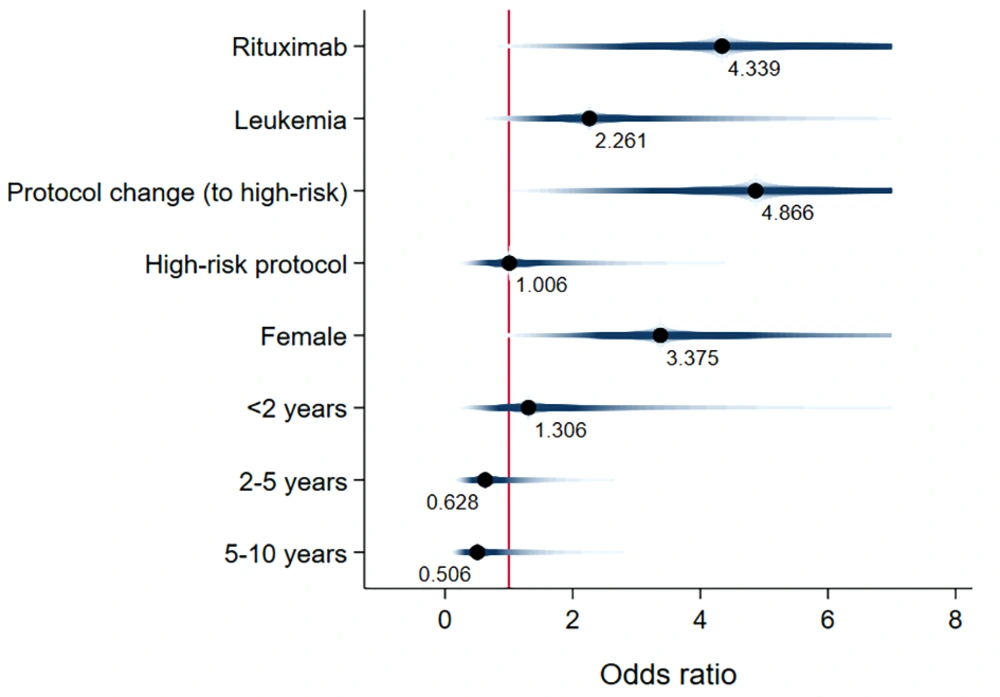
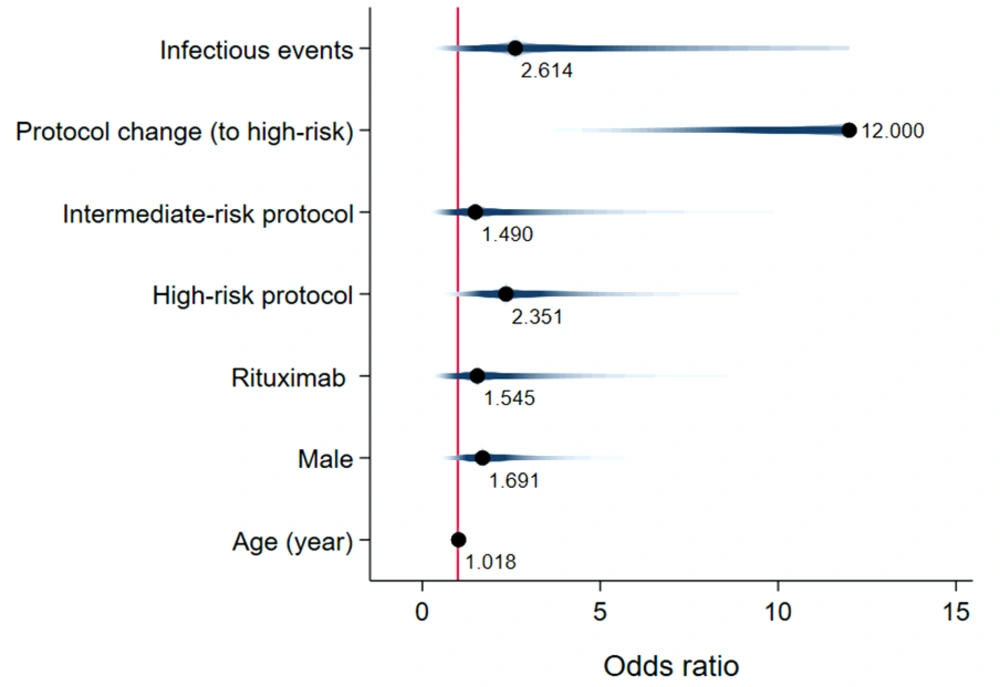
Unadjusted mean time-to-event data for death, FN, BSI, and IFI in both the exposed and unexposed groups are summarized in Table 2 and Figure 3. The adjusted effects of RTX treatment on patient outcomes were analyzed using Cox proportional hazards models. RTX was associated with an increased hazard risk of IFIs, death, FN events, and BSIs over a 36-month observation period (4.33 [1.21, 15.52], 3.26 [1.008, 10.59], 1.68 [0.83, 3.41], and 1.59 [0.278, 9.17], respectively) (Figures 4 - 7; Appendices 2 - 7).
| Variables and Study Groups | P-Value | Hazard Ratio [Exp (Beta)] | 95.0% CI for Exp (B) | |
|---|---|---|---|---|
| Lower | Upper | |||
| Death | ||||
| Exposed a | < 0.001 b | 17.19 | 12.37 | 22.01 |
| Unexposed | 28.68 | 26.40 | 30.96 | |
| BSI | ||||
| Exposed | 0.309 | 23.53 | 17.12 | 29.94 |
| Unexposed | 27.28 | 24.91 | 29.65 | |
| IFI | ||||
| Exposed | 0.039 b | 25.24 | 19.48 | 31.00 |
| Unexposed | 31.03 | 28.80 | 33.26 | |
| FN | ||||
| Exposed | 0.01 b | 7.55 | 3.40 | 11.69 |
| Unexposed | 12.32 | 10.03 | 14.60 | |
Abbreviations: BSI, bloodstream infection; IFI, invasive fungal infection; FN, febrile neutropenia.
a RTX-treated group.
b Statistically significant by log-rank test.
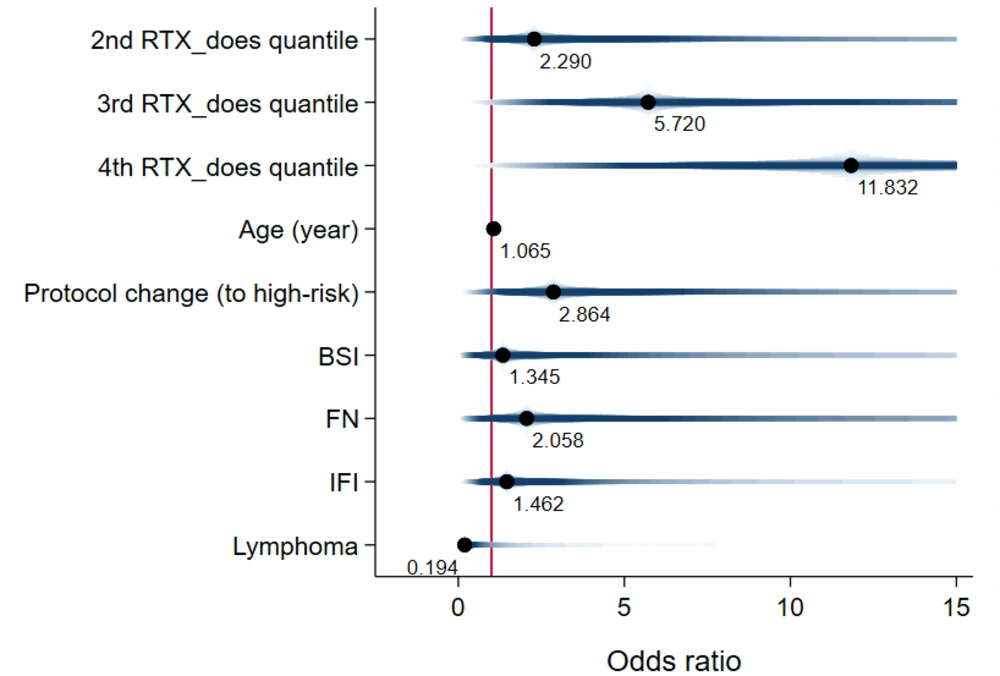
The total RTX dose administered was also linked to poorer patient outcomes. The adjusted odds of death increased across the second, third, and fourth quartiles of the total RTX dose (2.28 [0.12, 41.56], 5.71 [0.67, 48.24], and 11.83 [0.95, 146.16], respectively) (Figure 8). Similarly, the adjusted odds of infectious events rose with the total RTX dose across the second, third, and fourth quartiles (2.45 [0.19, 30.99], 3.17 [0.21, 47.18], and 6.37 [0.74, 54.19], respectively) (Figure 9). For more information, refer to Appendices 6 and 7.
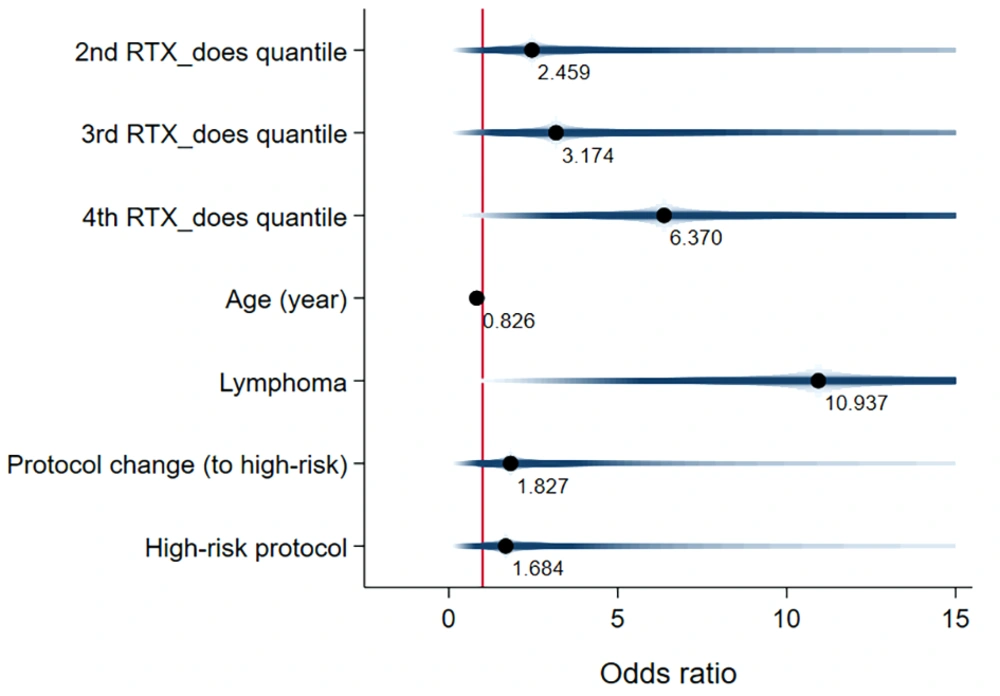
The logistic regression model's results also indicated that the adjusted effect of RTX similarly affected patient survival, showing an increased odds of death (OR [95%CI]: 1.54 [0.41, 5.69]) (Figure 10). Finally, the estimated one-year, two-year, and three-year cumulative proportional survival rates were 77%, 44%, and 33%, respectively (Appendix 9).
5. Discussion
This study extensively examined the effect of RTX on infectious complications and the survival of children with malignancy. The findings highlight the significant clinical impact of RTX treatment on the incidence of FN events, which are often associated with more severe infectious outcomes (19). Our research supports the notion that the risk of bacterial and fungal infections escalates alongside the risk of FN. Thus, additional preventive measures are recommended for patients not traditionally viewed as being at high risk for fungal infections, such as children with CD20+ lymphoma (19, 20). Notably, Cox proportional hazards models revealed that fungal infections posed the highest adjusted hazard risk, nearly 2.7 times greater than the adjusted risk for bacterial BSI (4.3 vs. 1.6). Later in this section, we will delve into existing research on the influence of RTX treatment on bacterial BSI risk, noting the scarcity of information regarding its effect on IFIs.
The data on the impact of RTX on infectious complications and the survival of patients receiving immunosuppressive therapy are mixed. While some studies align with our findings, others remain inconclusive, suggesting that our study could contribute additional insights for managing cancer patients undergoing RTX treatment concurrently. Lee McAtee et al. reported that among pediatric patients treated with RTX, there was an increased risk of infections associated with the simultaneous use of chemotherapy (adjusted hazard ratio [aHR], 2.35; 95% CI, 1.33 - 4.04; P < 0.001). Infections were observed in 47.9% (224/468) of cases; severe infections occurred in 17.9% (84/468), and death in 0.6% (3/468). They noted that B-cell recovery (CD19+ or CD20+ cell count normalization) typically took about 9.0 months (interquartile range, 5.9 - 14.4 months) after RTX treatment (17).
Lanini et al. found that RTX significantly elevated the risk of both infection and neutropenia in patients with lymphoma or other hematological malignancies (21). A prospective randomized trial by Van Oers et al. indicated a higher frequency of infections and neutropenia among RTX-treated patients compared to those not exposed to RTX, offering more comprehensive data on the subject (22). Additionally, a study by Nissen et al. showed a notably higher incidence of infectious complications in patients receiving a combination of RTX and chemotherapy versus those on RTX monotherapy. They reported no significant differences in the number of RTX courses or cumulative RTX doses between treatment episodes with and without infections (14).
In contrast, a meta-analysis indicated that incorporating RTX into standard chemotherapy protocols did not alter the overall risk of severe infections or survival (13). Similarly, a systematic review of 9 randomized controlled trials (RCTs) by Rafailidis et al. found no significant increase in the incidence of infections among those treated with RTX for B-cell non-Hodgkin lymphoma (NHL) (23). These divergent findings could stem from the absence of an unexposed control group, a limited follow-up period, or a small sample size. Additionally, we identified two other studies, one by Witzens-Harig et al. focusing on adult patients with follicular lymphoma (24) and another by de Souza et al. examining adults with NHL (25). Witzens-Harig et al.'s study highlighted that RTX did not lead to severe or uncommon infections in adult follicular lymphoma patients. Conversely, de Souza et al. reported an increased vulnerability to respiratory infections in adults treated with RTX for NHL.
Research on the clinical effects of RTX on the risk of infectious outcomes in patients with immune thrombocytopenic purpura (ITP) is limited. A systematic review by Arnold et al. reported that 2.3% of patients developed severe infections, but it's important to note that many of these patients were also taking other immunosuppressants concurrently, making it difficult to attribute these outcomes solely to RTX (26). Stabler et al. found an increased risk of infectious events following RTX treatment in patients with autoimmune diseases, including autoimmune cytopenias, with ITP patients comprising 18% (40/221) of the study population. However, no specific analysis focused on pediatric ITP patients was conducted. Infectious complications occurred in 19% (42/221) of the cases, with bacterial infections being the most common, followed by fungal infections (55% and 12%, respectively). Identified risk factors for severe infectious events included age, a history of diabetes, a history of cancer, concurrent steroid treatment, and a low CD4 lymphocyte count at the start of RTX treatment (27).
Our study stands out as one of the few comprehensive investigations into the effects of RTX in pediatric patients with cancer and hematologic disorders, particularly focusing on various independent variables, notably the characteristics of immunosuppressive medications used.
This study faced several limitations. Firstly, a larger sample size would bolster our findings, as our study's sample size was relatively small. Our cohort also included a limited number of ITP patients, potentially skewing the results and necessitating cautious interpretation of these patients' outcomes. Secondly, the potential for selection bias must be considered, as the study predominantly included patients with CD20-positive B-cell non-Hodgkin lymphoma in the exposed group, not fully representing the target population. However, we sought to mitigate this bias by using adjusted statistical models that included underlying diseases as a covariate. Thirdly, as with other cohort studies, there may be unknown background risks or residual confounders affecting both the exposed and unexposed groups.
The majority of knowledge about RTX clinical effects on patients undergoing immunosuppressive chemotherapy comes from studies on adults. Additionally, the impact of simultaneous RTX treatment alongside other anticancer medications in children remains largely unexplored. Consequently, the findings of this study shed light on the specific and quantifiable effects of RTX on pediatric cancer patients. Our analyses offer a nuanced understanding of how the intensity and duration of RTX treatment influence both patient survival and the incidence of infection complications.
5.1. Conclusions
The addition of RTX to the treatment regimen of children receiving immunosuppressive chemotherapy for hematologic malignancies, especially CD20-positive B-cell lymphoma, has shown both additive and dose-dependent effects on clinical outcomes. However, due to potential uncontrolled biases and unrecognized background risks, these results should be approached with caution.