1. Background
Upper urinary tract infection (UTI) is one of the most common serious bacterial infections in febrile infants and young children. Early diagnosis and management of febrile UTI are essential to prevent long-term complications, such as renal scarring, hypertension, proteinuria, and chronic kidney disease. However, the accurate diagnosis and management of acute pyelonephritis (APN) pose a clinical challenge due to the lack of specific clinical and laboratory findings, especially in infants and young children. Identifying the causative organism through urine culture takes about 2 - 3 days and may yield false-positive or false-negative results. Additionally, urinalysis and inflammatory markers, such as fever, white blood cell (WBC) count, ESR, and CRP, have limited sensitivity and specificity for differentiating APN from other bacterial infections (1).
Recently, serum D-dimer has been explored as a diagnostic biomarker for APN, with conflicting results. Serum D-dimer is a product of fibrin degradation produced during activation of the coagulation system. It is a simple, accessible test that reflects coagulation system activation and the severity of the patient’s inflammatory response. Additionally, serum D-dimer acts as an acute-phase reactant that stimulates an inflammatory response by activating neutrophils and monocytes and increases cytokine levels (IL1 and IL6) during inflammatory disorders. It has been suggested as a significant prognostic marker in patients with infectious diseases and sepsis (2, 3).
2. Objectives
This study aimed to investigate the value of serum D-dimer as a biomarker for acute febrile UTI compared to other acute bacterial febrile infections. Additionally, the study will examine serum D-dimer alterations in vesicoureteral reflux (VUR), an important risk factor for UTI.
3. Methods
3.1. Study Design and Data Collection
A cross-sectional study was conducted on a random sample of 94 febrile children with either UTI (N = 47) or other bacterial infections (N = 47), admitted to two tertiary children’s hospitals in Tehran and Babol between 2021 and 2022. Inclusion criteria consisted of children with documented UTI (positive urine culture) or other bacterial infections, such as pneumonia, meningitis, osteomyelitis, sinusitis, or mastoiditis, confirmed by clinical manifestations in addition to hematologic, microbiologic, radiologic, or radioisotopic findings. Patients with decreased renal function were excluded from the study.
Demographic and clinical characteristics, including age and gender, were recorded for each patient. Blood samples were obtained for laboratory investigations, including hemoglobin (Hgb), WBC count, platelets, ESR, CRP, and D-dimer levels. D-dimer was measured at the time of admission before antibacterial treatment using a latex immunoturbidometric assay (Sclavo D-Dimer Kit, Sclavo Diagnostics International, Italy), with values ≥ 500 ng/mL considered positive.
Acute pyelonephritis was defined as fever (body temperature ≥ 38°C) with pyuria (≥ 5 WBC/hpf), elevated ESR or CRP, and a positive urine culture (≥ 105 CFU/mL in a bag collection, ≥ 104 CFU/mL in a catheterized sample, or any growth in a suprapubic aspiration) (1).
Renal ultrasound was performed for all patients with APN by a single radiologist. Abnormal sonographic findings indicative of APN included renal enlargement, increased renal parenchymal echogenicity, and absence of corticomedullary differentiation. Voiding cystourethrography (VCUG) was conducted to investigate VUR in patients with abnormal renal ultrasound findings, atypical UTI, or delayed medical response, and VUR was classified into grades I-V, according to the International Reflux Study in Children.
3.2. Statistical Analysis
Statistical analyses were conducted using SPSS version 27.0 for Windows (SPSS Inc., Chicago, IL, USA) and MedCalc 15.4 (MedCalc software, Belgium). Descriptive statistics, including mean, standard deviation, frequency, and percentages, were used to present the results. Data normality was assessed using the Kolmogorov-Smirnov test. Variables with normal distribution were compared using the two-sample t-test, while non-normally distributed variables were analyzed using the Mann-Whitney test. Categorical variables were compared using chi-square and Fisher’s exact tests.
The relationship between variables was examined using the Spearman correlation test. Binary logistic regression analysis was performed to identify independent predictive factors for APN. The area under the curve (AUC) values from the receiver operating characteristic (ROC) curve were used to predict APN and VUR. A P-value ≤ 0.05 was considered statistically significant.
3.3. Ethical Approval
The study received ethics approval (IR.SBMU.MSP.REC.1400.809) from the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all legal guardians.
4. Results
A total of 94 children (47 with UTI confirmed by positive urine culture and 47 with other bacterial febrile infections and negative urine culture), aged 2 to 123 months, were included in this study. Females significantly outnumbered males (P = 0.013). Urinary incontinence was the most common symptom in children with UTI (61.7%), followed by urgency (31.9%), malodorous urine (27%), and dysuria (4.3%). Approximately 78.7% and 93.6% of patients with UTI exhibited microscopic hematuria and bacteriuria, respectively. E.coli was the most common causative organism of UTI.
Serum D-dimer levels were elevated in both children with febrile UTI and those with other febrile infections, with no significant difference observed between the groups. Significant differences were found in WBC (P = 0.047), Hgb (P = 0.035), bacteriuria (P < 0.001), and microscopic hematuria (P = 0.044) among the study participants (Table 1). Eleven children had VUR (3 right, 3 left, and 5 bilateral). Serum D-dimer levels showed no significant difference in patients with different grades of VUR compared to those without VUR.
| Variables | With UTI | Without UTI | P-Value |
|---|---|---|---|
| Age (m) | 45.04 ± 38.33 (2 - 132m) | 33.88 ± 30.66 (3 - 120m) | 0.007 |
| Gender (M/F) | 2/45 (4.3/95.7) | 10/37 (21.3/78.7) | 0.013 |
| Temperature (°C) | 37.58 ± 0.85 | 37.73 ± 0.87 | 0.415 |
| WBC (mm3) | 15.60 ± 6.29 | 13.41 ± 6.88 | 0.047 |
| ESR (mm/h) | 56.57 ± 31.60 | 49.98 ± 33.33 | 0.327 |
| Hgb (g/dL) | 10.89 ± 1.10 | 10.32 ± 1.47 | 0.035 |
| PLT (hpf) | 374000.07 ± 137.29 | 406000.49 ± 196.90 | 0.667 |
| CRP (mg/dL) | 62.85 ± 58.96 | 55.88 ± 50.00 | 0.725 |
| Urine PH | 5.66 ± 0.91 | 5.60 ± 1.11 | 0.930 |
| D-dimer | 575.72 ± 423.19 | 540.81 ± 448.88 | 0.327 |
Abbreviations: m, month; M, male; F, female; UTI, urinary tract infection; Hgb, hemoglobin; WBC, white blood cell.
a Values are expressed as mean ± SD.
Binary logistic regression analysis indicated that Hgb (OR: 1.731, 95% CI: 1.092 - 2.744, P = 0.019) was an independent predictor of APN, whereas serum D-dimer was not (Table 2).
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| WBC (mm3) | 1.055 (0.992 - 1.123) | 0.089 | 1.053 (0.979 - 1.132) | 0.162 |
| CRP (mg/dL) | 1.002 (0.995 - 1.010) | 0.535 | 1.001 (0.993 - 1.009) | 0.866 |
| Urine PH | 1.065(0.713 - 1.591) | 0.760 | 1.112 (0.720 - 1.718) | 0.631 |
| D-dimer | 1.000 (0.999 - 1.001) | 0.696 | 1.000 (0.999 - 1.001) | 0.880 |
Abbreviation: WBC, white blood cell.
A significant positive correlation was observed between serum D-dimer and ESR (r = 0.333, P = 0.001), while a significant negative correlation was noted between serum D-dimer and Hgb (r = 0.267, P = 0.010) (Table 3).
| D-dimer | Correlation | P-Value |
|---|---|---|
| ESR | 0.333 | 0.001 |
| WBC | 0.172 | 0.099 |
| Hgb | -0.267 | 0.010 |
| PLT | 0.112 | 0.286 |
| CRP | 0.153 | 0.141 |
| Urine pH | -0.119 | 0.253 |
Abbreviations: Hgb, hemoglobin; WBC, white blood cell.
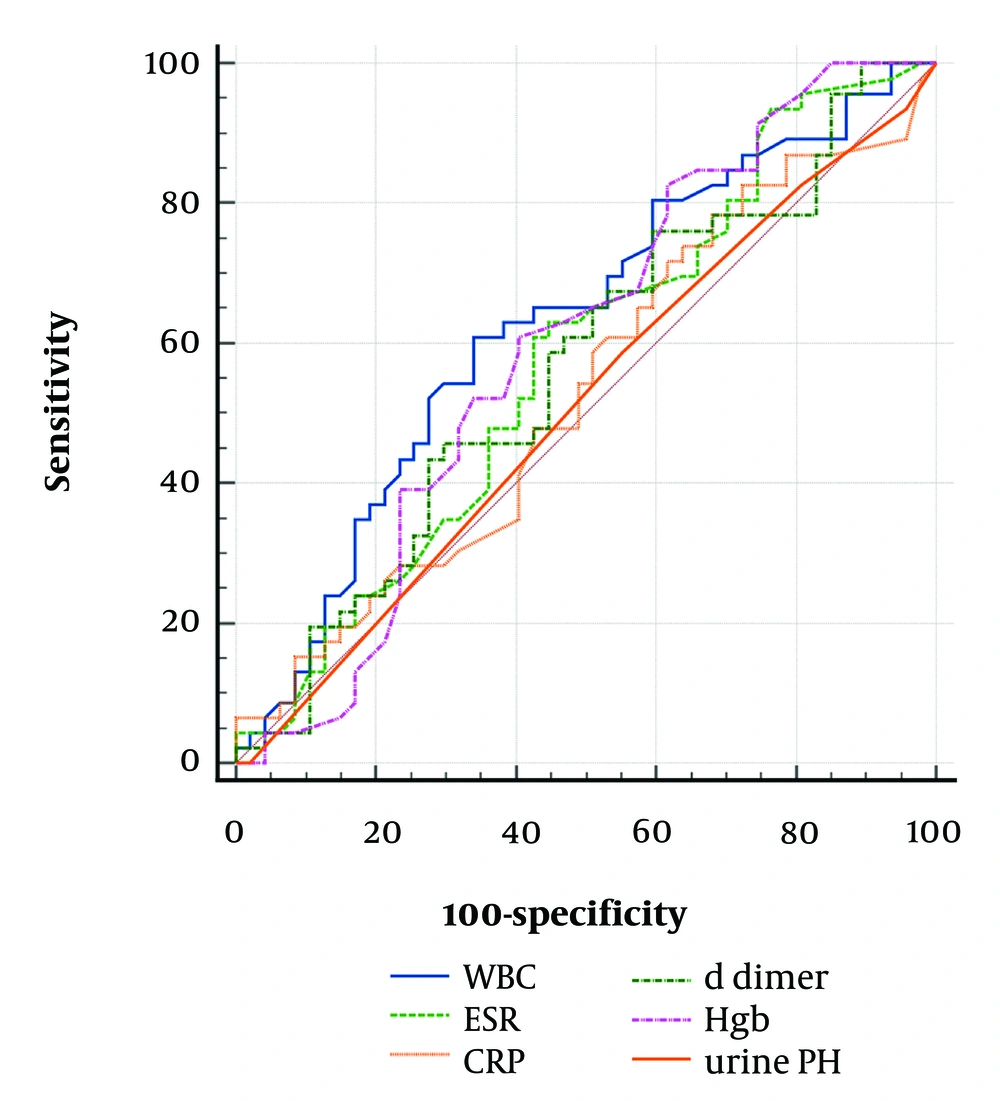
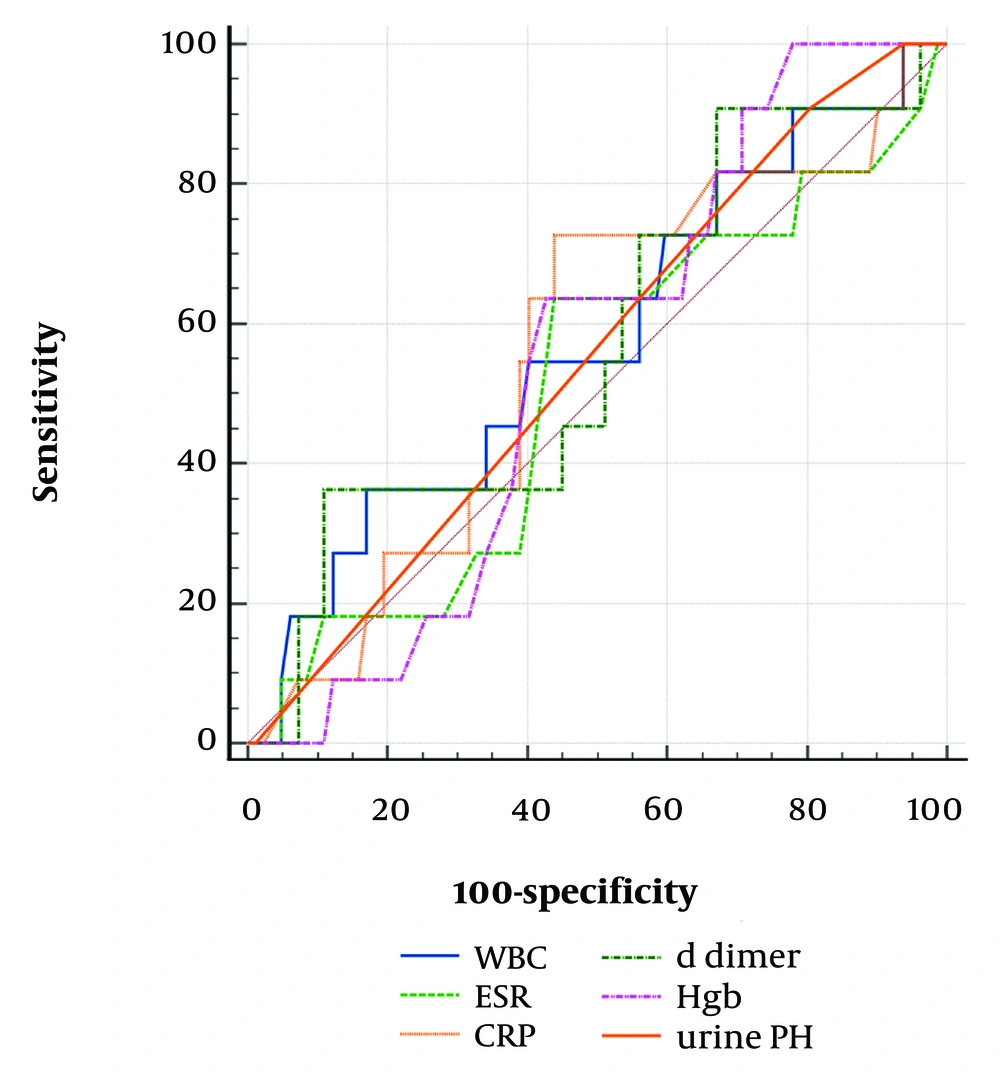
The AUC for Hgb (0.621, P = 0.041) was higher than for WBC (0.619, P = 0.042). The cutoff values of Hgb > 9.7 g/dL (P = 0.041) and WBC >13,400/mm³ (P = 0.042) were identified as accurate predictors of APN. However, serum D-dimer was not an accurate predictor of APN (P = 0.328) and VUR (P = 0.439) (Tables 4 and 5, Figures 1 and 2).
| Variables | Cutoff | Sensitivity | Specificity | AUC | 95% CI | Standard Error | P-Value |
|---|---|---|---|---|---|---|---|
| WBC | 13.4 | 59.6 | 66.0 | 0.619 | 0.513 - 0.717 | 0.058 | 0.042 |
| ESR | 45 | 61.7 | 55.3 | 0.562 | 0.456 - 0.665 | 0.060 | 0.296 |
| CRP | 18 | 76.6 | 31.9 | 0.521 | 0.416 - 0.625 | 0.061 | 0.727 |
| D-dimer | 497 | 44.7 | 70.2 | 0.559 | 0.452- 0.661 | 0.060 | 0.328 |
| Hgb | 9.7 | 80.3 | 40. 4 | 0.621 | 0.513 - 0.717 | 0.059 | 0.041 |
| Urine PH | 5 | 57.4 | 44.7 | 0.505 | 0.400 - 0.610 | 0.054 | 0.930 |
| D-dimer + VUR | 844 | 36.4 | 89.2 | 0.574 | 0.468-0.675 | 0.096 | 0.439 |
Abbreviations: AUC, area under the curve; WBC, white blood cell; Hgb, hemoglobin; VUR, vesicoureteral reflux.
| Variables | Cutoff | Sensitivity | Specificity | AUC | 95% CI | Standard Error | P-Value |
|---|---|---|---|---|---|---|---|
| Malodor urine | 382 | 51.1 | 69.4 | 0.578 | 0.460 - 0.696 | 0.060 | 0.193 |
| Urgency | 416 | 69.2 | 63.2 | 0.671 | 0.542 - 0.801 | 0.066 | 0.009 |
| Urinary incontinency | 386 | 65.0 | 52.9 | 0.570 | 0.446 - 0.693 | 0.063 | 0.269 |
Abbreviations: AUC, area under the curve.
5. Discussion
A persistent inflammatory response with cytokine activation or expression of tissue factors is a primary cause of hypercoagulation in various inflammatory or infectious disorders. The inflammatory process and coagulation pathway play significant roles in the body’s response to bacterial infections. D-dimer, a biomarker of fibrin degradation and coagulation, has been linked to the activation of the coagulation system and decreased urinary excretion, which are considered the main causes of elevated serum D-dimer levels in patients with renal disorders (2, 4). However, all of our study participants had normal GFR, as assessed by serum creatinine.
We found that children with APN had significantly higher WBC, Hgb, and microscopic hematuria compared to those with other bacterial infections. Notably, Hgb > 9.7 g/dL was a reliable predictor of APN, with an acceptable sensitivity of 80%. Additionally, WBC > 13,400/mm³ was found to be an accurate and meaningful variable for predicting acute febrile UTI, though with lower sensitivity and specificity.
Based on the cutoff values, serum D-dimer levels were elevated across all participants with different bacterial febrile infections. However, D-dimer was not a significant differentiating factor for APN compared to other bacterial febrile infections, suggesting it serves as a general acute inflammatory biomarker. Furthermore, the cutoff value of serum D-dimer was higher in cases of APN complicated by VUR than in those without VUR, possibly reflecting a secondary response to bacterial inflammation in VUR.
Similarly, Esteghamati et al. evaluated plasma D-dimer levels in two separate studies, showing increased D-dimer in 16.3% of children with febrile UTI, regardless of the isolated organism. They suggested that serum D-dimer measurement is an appropriate test for diagnosing UTI with low false negative rate (4). In a subsequent study, they reported a significant positive correlation between serum D-dimer and ESR and CRP, but not with age or gender in children with febrile UTI (5). In our study, we also found a positive correlation between serum D-dimer and ESR, underscoring D-dimer’s role as an inflammatory marker in bacterial infections.
In the report by Lee et al., the AUC of serum D-dimer was found to be superior to other inflammatory biomarkers, such as WBC and ESR, but inferior to CRP for predicting APN. They observed higher serum D-dimer levels in APN compared to lower UTIs, suggesting D-dimer as an acute-phase inflammatory biomarker in infants with APN. Additionally, they identified it as a significant predictor of VUR (2). In our study, we also reported elevated serum D-dimer in children with acute febrile UTI, with a higher cutoff in those with VUR.
Mu et al. demonstrated that increased serum D-dimer acts as an independent risk factor for UTI in patients with intracranial hemorrhage, indicating its role as an inflammatory biomarker for UTI (6). Similarly, Rodelo et al. suggested that D-dimer serves as a prognostic marker in patients with inflammation and septicemia (3).
Consistent with our findings, Lins et al. reported significantly higher serum D-dimer levels in patients with conditions such as pneumonia, bronchitis, and UTI compared to a healthy control group (7).
These findings suggest that serum D-dimer functions as an inflammatory biomarker across various bacterial febrile infections, including APN, but lacks specificity as a diagnostic marker for febrile UTI compared to other bacterial infections.
5.1. Conclusions
Serum D-dimer levels increased in children with APN and other bacterial febrile infections in our study. It was identified as a nonspecific inflammatory biomarker for various bacterial infections but not as a reliable diagnostic test for febrile UTI in cases with diagnostic ambiguity. However, due to the relatively small sample size in our study, future research with larger patient groups is recommended to better assess the predictive value of serum D-dimer in children with acute febrile UTI, in comparison to lower UTI and other bacterial infections.