1. Background
Given the COVID-19 outbreak, there has been increased focus on understanding the virus's impact on infants. Notably, many women delayed pregnancy during the pandemic (1). Concerns rose as the virus began affecting infants more frequently, with documented cases of infant involvement (2, 3). As COVID-19 cases increased, observations indicated that infants generally experienced milder symptoms than adults (4). This raised questions about the necessity of hospitalization for all COVID-19-infected infants and whether it’s possible to predict disease severity in these cases to avoid unnecessary hospital admissions (5). While there are studies on predicting COVID-19 severity in adults and children, research on this topic specifically for infants remains limited (6-8). A recent study highlighted that while postnatal COVID-19 infection can occur in newborns, most cases resolve favorably (9). In this study, we evaluated the severity of infection in 64 COVID-19-positive infants under three months of age using demographic, laboratory, and radiologic data.
2. Objectives
The objective is to identify correlations between this data and disease severity to improve decision-making for these infants.
3. Methods
3.1. Study Design
This cross-sectional study was conducted on all neonates and infants under three months of age who were admitted to the neonatal intensive care unit (NICU) or neonatal ward of the Children's Medical Center in Tehran, Iran, between October 2020 and March 2022. Inclusion criteria included neonates or infants younger than three months with a positive PCR test for COVID-19 and admission to the NICU or neonatal ward during the study period. We excluded neonates with positive blood cultures to remove this confounding factor, as it could influence disease severity. Neonates with incomplete medical records were also excluded.
The variables included in the study were birth weight, gestational age, current weight, sex, chronological age, underlying medical conditions, respiratory distress, oxygen requirements, respiratory support, hospitalization duration, radiographic and laboratory data [including white blood cell (WBC) count and differential counts, platelet count, hemoglobin level, vitamin D level, ferritin level, lactate dehydrogenase (LDH) level, fibrinogen level, D-dimer level, C-reactive protein (CRP) level, liver function tests (LFT), and the infants’ outcomes]. Quantitative RT-PCR samples were obtained from nasopharyngeal swabs using the Pishtaz Teb Coronavirus RT-PCR kit (98001). Radiographic findings such as bronchial wall thickening, diffuse ground-glass opacity, and peripheral opacity were noted, with the radiologist blinded to disease severity.
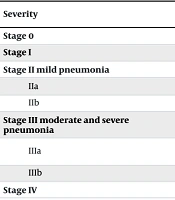
Infection severity was categorized based on criteria from a prior study by Qiu et al. (10) (Table 1). All information was recorded in a pre-structured questionnaire, and the associations between disease severity and the collected variables were analyzed.
| Variables | Values |
|---|---|
| Case number | 64 (100) |
| Sex (male) | 23 (33.8) |
| Age of admission (day) | 27.97 ± 19.445 |
| Birth gestational age (w) | 37.7 (31 - 40) |
| Current weight (gr) | 3952 (2010 - 6500) |
| Preterm | 8 (11.9) |
| Hospitalization (day) | 4.60 ± 3 |
| Fever | 42 (62.7) |
| Cough | 23 (34.3) |
| Diarrhea | 15 (22.38) |
| Jaundice b | 7 (10.4) |
| Vomiting | 12 (17.9) |
| Apnea | 4 (6) |
| Convulsion | 1 (1.4) |
| Mottling | 0 (0) |
| Death | 0 (0) |
aValues are expressed as No. (%) or mean ± SD unless otherwise indicated.
b Jaundice refers to bilirubin at the limit of phototherapy.
3.2. Statistical Analysis
We performed statistical analysis using the Kolmogorov-Smirnov test to assess data normality. For data following a normal distribution, continuous data were presented as mean ± standard deviation, and qualitative data as frequency. For non-normally distributed data, we reported the interquartile range and used non-parametric tests. We applied a Binary Logistic Regression model to explore the relationship between laboratory data, clinical symptoms, and disease severity in infants. Stages 0 and I were grouped as "mild," while the remaining stages were categorized as "moderate to severe." Severity served as the dependent variable, and we calculated the odds ratio [EXP (B)] for each factor. Qualitative variables were compared using the chi-square test, with a P-value < 0.05 considered statistically significant. Data analysis was conducted using SPSS version 16.
3.3. Ethics
This study received approval from the Ethics Committee of Tehran University of Medical Science (ethics code: IR.TUMS.CHMC.REC.1400.187). All data were collected from patient files in the archives, and data coding was used to protect patient confidentiality.
4. Results
In this cross-sectional study, 64 COVID-19 positive infants were evaluated. Eleven patients were preterm, while the remaining were born at term. The average age of the infants was 27 days, with 11 patients admitted beyond 28 days of age and an age range extending up to 75 days. The average hospitalization duration was 4.6 days, ranging up to 7 days. Table 1 presents the demographic and clinical data of the studied infants. Based on infection severity, most COVID-19 positive neonates were categorized as stage IIb (43.75%), with none reaching stage IV (Table 2).
| Severity | Findings | Values; N = 64 (100) |
|---|---|---|
| Stage 0 | Without symptom | 2 (3.1) |
| Stage I | Mild upper respiratory symptoms without radiographic involvement | 16 (25) |
| Stage II mild pneumonia | ||
| IIa | Mild respiratory distress or radiographic involvement without oxygen requirement | 15 (23.5) |
| IIb | Mild respiratory distress or radiographic involvement with free flow oxygen requirement | 28 (43.75) |
| Stage III moderate and severe pneumonia | ||
| IIIa | Moderate and severe respiratory distress and radiographic involvement with non-invasive respiratory support | 1 (1.55) |
| IIIb | Moderate and severe pneumonia and radiographic involvement with invasive respiratory support | 2 (3.1) |
| Stage IV | Critical illness, septic shock and end-organ failure | 0 |
a Values are expressed as No. (%).
Table 3 displays the laboratory results. Our findings indicated a significant association between lower gestational age and increased severity (P-value = 0.02), with an odds ratio of 0.54. Disease severity also correlated with lower birth weight, showing statistical significance (P-value = 0.04) but with an odds ratio of 1 (Table 4). The highest severity observed in newborns delivered at 38-39 weeks gestation was classified as stage IIb. Notably, none of the patients reached stage IV, and the two infants with a severity level of IIIb were both born at 34 weeks gestation. All four cases with underlying conditions (including two cases of patent ductus arteriosus ligation, one case of congenital adrenal hyperplasia, and one case of severe laryngomalacia) exhibited a severity level of IIb or higher. However, due to the small number of cases with underlying disease, it was not possible to test the relationship reliably. Additionally, laboratory data from Table 3 were collected and analyzed, revealing no significant correlation between most blood laboratory values and COVID-19 severity in our study, except for WBC (P-value = 0.03). We also identified a significant correlation between cough symptoms and disease severity, with a P-value of 0.001 (Table 4).
| Tests | Mean ± SD | Range |
| Liver function tests | ||
| AST (IU/L) | 59.7 ± 21.45 | (16 - 114) |
| ALT (IU/L) | 33.8 ± 30.5 | (5 - 71) |
| PT (sec) | 16.45 ± 2.75 | (13 - 26.8) |
| PTT (sec) | 41.97 ± 12.74 | (30 - 89) |
| Renal tests | ||
| BUN (mg/dL) | 12.35 ± 18.9 | (2 - 93) |
| Cr (mg/dL) | 0.77 ± .1.3 | (0.2 - 1.4) |
| Inflammatory | ||
| CRP (mg/L) | 12.07 ± 25 | (0.5 - 120) |
| LDH (u/L) | 556 ± 74 | (295 - 889) |
| Ferritin (μg/L) | 610 ± 989 | (39 - 2090) |
| Coagulation | ||
| D-dimer (ng/mL) | 855 ± 159 | (10 - 4138) |
| Fibrinogen (mg/dL) | 281 ± 96 | (110 - 640) |
| Hematologic | ||
| WBC (μL) | 7515 ± 3213 | (1374 - 19700) |
| Neutrophil count (μL) | 1766 ± 1034 | (94 - 5226) |
| Lymphocyte count (Μl) | 4149 ± 2067 | (398 - 9456) |
| Vit D (ng/mL) | 39.58 ± 16 | (21 - 88) |
Abbreviations: CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; PTT, partial thromboplastin time; BUN, blood urea nitrogen; Cr, creatinine; LDH, lactate dehydrogenase; WBC, white blood cell.
| Variables | OR | 95%CI | P-Value |
|---|---|---|---|
| Lab data, N = 64 | |||
| WBC | 0.9 | 0.9, 1 | 0.03 |
| PLT | 1 | 1, 1 | 0.16 |
| Hb | 0.9 | 0.6, 1.1 | 0.5 |
| CRP | 1 | 0.95, 1.1 | 0.6 |
| LDH | 0.9 | 0.9, 1 | 0.2 |
| Ferritin | 1 | 0.9, 1 | 0.6 |
| D-dimer | 1 | 1, 1 | 0.1 |
| AST | 1 | 0.9, 1.2 | 0.2 |
| ALT | 0.9 | 0.8, 1.1 | 0.7 |
| Vit d | 1 | 0.9, 1.1 | 0.6 |
| BW | 1 | 1, 1 | 0.04 |
| GA | 0.54 | 0.32, 0.92 | 0.02 |
| Age at admission | 0.9 | 0.9, 1 | 0.1 |
| CW | 1 | 0.9, 1 | 0.9 |
| Symptoms | |||
| Vomiting | 0.7 | 0.17, 3.1 | 0.6 |
| Cough | 0.02 | 0.0, 0.1 | 0.001 |
| Poor feeding | 0.7 | 0.15, 3.2 | 0.6 |
| Diarrhea | 0.5 | 0.2, 1.3 | 0.2 |
| NEC | 1,5 | 0.2, 9 | 0.6 |
| RDS | 0.4 | 0.1, 1.8 | 0.2 |
| Fever | 0.2 | 0.05, 1.1 | 0.07 |
Abbreviations: WBC, white blood cell; LDH, lactate dehydrogenase; CRP, C-reactive protein.
Additionally, the laboratory data in Table 3 were analyzed, showing no significant correlation between most blood laboratory values and COVID-19 severity, except for WBC count (P-value = 0.03). A significant correlation was also found between cough symptoms and disease severity, with a P-value of 0.001 (Table 4).
5. Discussion
The clinical manifestations of COVID-19 in infants can vary significantly, ranging from asymptomatic or mild respiratory symptoms to severe respiratory distress and multi-organ involvement (11). This article aimed to identify the risk factors contributing to the severity of COVID-19 infection in infants. Understanding these factors is crucial for early identification, appropriate monitoring, and management of infants at risk for severe COVID-19 infection.
The study reveals a correlation between increased severity of COVID-19 infection and lower birth weight and gestational age. However, to draw more definitive conclusions, studies with larger sample sizes are needed. Similarly, Steiner et al., in an observational study, reported that premature infants are particularly susceptible to viral infections and tend to be more severely affected (12).
We observed that neonates with COVID-19 infection had elevated levels of D-dimer. However, no thrombotic events were reported in these patients, and no antithrombotic medication was administered, consistent with findings by Yaman et al. (13). Additionally, we found no correlation between LFT levels and other markers, such as ESR, CRP, serum ferritin, and D-dimer. This contrasts with observations in adult COVID-19 patients, where LFT abnormalities are often associated with elevated levels of these markers (14). Sun et al., in an observational study, reported elevated CRP, procalcitonin, and LDH levels in eight children with severe COVID-19 (7). Similarly, Chao et al. documented high levels of CRP, procalcitonin, pro–B-type natriuretic peptide, and platelet counts in 67 children admitted to the ICU (15).
Some studies suggest that elevated procalcitonin, CRP, and neutrophil levels in COVID-19 patients are due to secondary infections, rather than the virus itself (6, 8). Liu et al., in a review, noted that COVID-19 in children can cause both neutropenia and neutrophilia, with disease severity linked to neutrophilia (16). Gracia et al. (as cited by Liu et al.) previously reported high CRP levels and lymphopenia in COVID-19 patients. Our study, however, found elevated CRP levels with decreased neutrophils, and no notable correlation between most blood laboratory values, aside from WBC count, and COVID-19 severity (16).
A cross-sectional study by Abdelrazic et al. identified a strong relationship between vitamin D deficiency and COVID-19 severity (17). In our study, we measured vitamin D levels in 36 patients with prolonged hospitalization and found no deficiency or insufficiency. Additionally, both neonates with a severity level of IIIb had sufficient vitamin D levels. Thus, our findings suggest no significant association between vitamin D deficiency and COVID-19 severity in this cohort.
Fever and cough, as previously noted (9, 13), were common clinical presentations among the patients in our study. Consistent with prior findings, the clinical presentation, disease course, and outcomes in infants with COVID-19 tended to be mild (18, 19). As shown in Table 1, no cases of critical illness or death were observed among the patients. Researchers continue to explore why infants typically experience milder COVID-19 symptoms than adults. One hypothesis is that the virus's entry receptor, ACE2, is less developed in young children, possibly reducing the virus's ability to infect them. Additionally, children's immune systems may elicit a less intense inflammatory response, which could contribute to a milder course of illness. Nonetheless, it’s essential to acknowledge that COVID-19 in children can still progress to moderate or severe forms in certain cases (20).
In our study, only two infants required invasive respiratory support, classified as severity level IIIb. In an observational cohort study, Qiu et al. compared mild and moderate COVID-19 in 36 infected children, finding that moderate cases showed elevated levels of fever, lymphopenia, procalcitonin, creatine kinase-MB, and D-dimer (10). We observed a significant association between cough and disease severity in our study. However, the low odds ratio indicated that there was no substantial difference between mild and moderate-to-severe disease in relation to cough symptoms.
Four cases in our study had underlying medical conditions, with all of these patients experiencing disease severity at stage IIb or higher. Among infants classified as stage IIIb, 100% had underlying conditions. However, given the limited number of cases, it is not possible to draw a reliable conclusion. In a study by Tezer and Bedir Demirdag on children, severe COVID-19 illness was reported to be more common in patients with underlying conditions (21). Similarly, Pawloska et al. observed that comorbidities increased both the risk of disease severity and the duration of hospitalization in infants, which aligns with our findings (22).
5.1. Limitations
The limitations of our study include the small sample size of patients with underlying conditions, which restricts the ability to draw conclusive results in this subgroup. Therefore, further research with larger cohorts is essential to validate and expand upon our findings.
5.2. Conclusions
Our study demonstrated that lower birth weight and gestational age were associated with increased disease severity in infants with COVID-19. Additionally, underlying conditions appeared to contribute to greater disease severity. While laboratory and clinical findings generally did not correlate significantly with disease severity, WBC and cough symptoms were notable exceptions. Thus, clinical evaluations, including gestational age and the presence of underlying conditions, are more effective factors in guiding decision-making. Further research is warranted to clarify the impact of these factors on COVID-19 severity in infants.