1. Background
Ascitic fluid infection (AFI) is the most common bacterial infection in patients with cirrhosis (1-3). Spontaneous bacterial peritonitis (SBP), a typical variant of AFI, is defined as ascitic fluid with a polymorphonuclear leukocyte (PMN) count ≥ 250 cells/mm³ and a positive ascitic fluid culture (2, 3). Bacterascites (BA), also known as monomicrobial non-neutrocytic bacterascites, is another variant of AFI, defined as a positive ascitic culture with a PMN count < 250 cells/mm³ (1, 4). Culture-negative neutrocytic ascites (CNNA) is another variant of AFI, defined as a negative ascitic culture with a PMN count > 250 cells/mm³ (5).
2. Objectives
3. Methods
3.1. Participants and Groups
A cross-sectional analytical study was conducted at a referral hospital affiliated with Shiraz University of Medical Sciences from June 2018 to September 2022. All cirrhotic patients with ascites were evaluated for the presence of AFI. Patients with AFI were divided into SBP, BA, and CNNA variants according to the criteria identified below. Non-AFI participants were also evaluated as a comparison group. Exclusion criteria included secondary peritonitis, history of antibiotic use in the past 14 days, and non-cooperative patients. Necessary variables, including gender, age, ethnicity, individual habits, clinical features, associated conditions, blood and ascitic fluid data, length of hospital stay (LOS), and in-hospital mortality, were recorded in a checklist. Finally, the clinical and laboratory characteristics, as well as the outcomes of the AFI variants, were compared.
3.2. Ethical Approval Statement
This study was conducted in accordance with the ethical declaration of Helsinki research and was approved by the ethics committee and institutional review board of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1398.483). Written informed consent was obtained from all participants.
3.3. Diagnosis of Ascetic Fluid Infection and its Variants
In all cirrhotic patients, diagnostic paracentesis of the abdomen was performed under sterile conditions within the first three hours of hospitalization. All ascitic samples were sent to the laboratory for evaluation of albumin, protein, culture, cell counts, and differentiation. For ascitic fluid culture, 10 milliliters of the sample were inoculated in a blood culture bottle (BD BACTEC, PEDS PLUS/F medium, Becton, Dickinson Co., USA) using the BD BACTEC 9240 system (Becton, Dickinson Co., USA).
Spontaneous bacterial peritonitis was defined as ascitic fluid PMN count ≥ 250 cells/mm³ and positive ascitic fluid culture. Bacterascites was defined as PMN count < 250 cells/mm³ and positive ascitic fluid culture. Culture-negative neutrocytic ascites was defined as PMN count ≥ 250 cells/mm³ and negative ascitic fluid culture.
3.4. Measurement of Laboratory Parameters of Blood Sample
Blood samples were taken from all participants to evaluate liver biochemical tests, complete blood count, albumin, protein, international normalized ratio (INR), partial thromboplastin time (PTT), blood urea nitrogen (BUN), and creatinine. The blood samples were transferred to the laboratory within an hour, and all laboratory parameters were tested according to international standards. Finally, the serum-ascites albumin gradient (SAAG) and model for end-stage liver disease (MELD) scores were calculated based on the laboratory results for all participants.
3.5. Statistical Analysis
The data was stored using IBM SPSS Statistics 25.0 software from Chicago, USA. A chi-square test was performed to compare qualitative data between groups. An independent sample t-test was used to compare quantitative variables between two groups. One-way ANOVA and the Kruskal-Wallis test were used to compare quantitative variables between three or more groups for a single independent variable, where appropriate. Kaplan-Meier curves and log-rank tests were used for survival analysis, comparing the AFI groups. Cox regression analysis was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) to evaluate the risk of various independent variables on hospital mortality. A P-value of less than 0.05 was considered statistically significant.
4. Results
A total of 466 patients were evaluated in this study, of which 132 (28.33%) had AFI and 334 (71.67%) did not have AFI. Among the AFI group, 64 (48.48%) had SBP, 43 (32.58%) had CNNA, and 25 (18.94%) had BA. The gender distribution was 313 (67.2%) male and 153 (32.8%) female patients. The mean age (SD) of the patients was 56.98 (14.80), with a range of 18 to 88 years. The most common associated symptoms were abdominal pain (98.7%) and peripheral edema (92.9%). Table 1 presents the demographic and clinical characteristics of the participants with and without AFI. The age of the AFI group was significantly lower than that of the non-AFI group. The AFI group had a significantly higher frequency of fever, peripheral edema, hepatic encephalopathy, hospital LOS, and mortality than the non-AFI group.
| Variables | With AFI | Without AFI | P-Value |
|---|---|---|---|
| Gender b | 0.165 | ||
| Male | 95 (72.0) | 218 (65.3) | |
| Female | 37 (28.0) | 116 (34.7) | |
| Age (y) c | 54.51 ± 13.95 | 57.96 ± 15.03 | 0.023 |
| Abdominal pain b | 129 (97.7) | 331 (99.1) | 0.358 |
| Jaundice b | 97 (73.5) | 230 (68.9) | 0.326 |
| Peripheral edema b | 129 (97.7) | 304 (91.0) | 0.011 |
| Fever b | 97 (73.5) | 105 (31.4) | < 0.001 |
| Nausea /vomiting b | 109 (82.6) | 265 (79.3) | 0.429 |
| Gastrointestinal bleeding b | 64 (48.5) | 142 (42.5) | 0.242 |
| Hepatic encephalopathy b | 60 (45.5) | 85 (25.4) | < 0.001 |
| Renal failure b | 45 (34.1) | 146 (43.7) | 0.057 |
| Hospital LOS (days); d | 32.30 ± 21.38 | 13.68 ± 15.32 | < 0.001 |
| Mortality b | 42 (31.8) | 50 (15.0) | < 0.001 |
Abbreviations: AFI, ascitic fluid infection; LOS, length of stay.
a Values are expressed as No. (%) or mean ± SD.
b Chi-square test.
ct-test.
d Mann-Whitney test.
As presented in Table 2, there were significant differences in abdominal pain, peripheral edema, fever, nausea/vomiting, hepatic encephalopathy, and renal failure among the three AFI groups.
| Variables | SBP (n = 64) | CNNA (n = 43) | BA (n = 25) | P-Value |
|---|---|---|---|---|
| Gender b | <0.001 | |||
| Male | 57 (89.1) | 28 (65.1) | 10 (40.0) | |
| Female | 7 (10.9) | 15 (34.9) | 15 (60.0) | |
| Age (y) c | 56.17 ± 13.25 | 50.72 ± 15.054 | 56.76 ± 12.891 | 0.093 |
| Abdominal pain b | 64 (100.0) | 40 (93.0) | 25 (100.0) | 0.042 |
| Jaundice b | 53 (82.8) | 28 (65.1) | 16 (64.0) | 0.062 |
| Peripheral edema b | 64 (100.0) | 43 (100.0) | 22 (88.0) | 0.001 |
| Fever b | 50 (78.1) | 34 (79.1) | 13 (52.0) | 0.026 |
| Nausea /vomiting b | 56 (87.5) | 37 (86.0) | 16 (64.0) | 0.024 |
| Hepatic encephalopathy b | 35 (54.7) | 22 (51.2) | 3 (12.0) | 0.001 |
| Gastrointestinal bleeding b | 33 (51.6) | 16 (37.2) | 15 (60.0) | 0.153 |
| Renal failure b | 28 (43.8) | 7 (16.3) | 10 (40.0) | 0.010 |
Abbreviations: SBP, spontaneous bacterial peritonitis; BA, bacterascites; CNNA, culture negative neutrocytic ascites.
a Values are expressed as No. (%) or mean ± SD.
b Chi-square Test.
c One-way ANOVA.
There were also significant differences in laboratory parameters such as hemoglobin, INR, bilirubin, BUN, MELD score, ascitic fluid protein, and albumin. Table 3 displays the comparison of laboratory parameters among the three AFI groups. Escherichia coli was the most common bacteria responsible for AFI, followed by Staphylococcus and Enterobacter.
| Variables | SBP (n = 64) | CNNA (n = 43) | BA (n = 25) | P-Value |
|---|---|---|---|---|
| White blood cells; μL b | 10518.75 ± 5002.06 | 9169.77 ± 4906.34 | 8416.00 ± 4925.33 | 0.132 |
| Hemoglobin; g/dL c | 9.06 ± 2.63 | 9.77 ± 2.52 | 10.49 ± 2.25 | 0.047 |
| Platelet; μL b | 125343.75 ±206404.92 | 85837.21 ± 25801.38 | 75000.00 ± 26702.06 | 0.365 |
| PTT; seconds b | 45.48 ± 18.96 | 45.37 ±22.26 | 37.76 ±8.45 | 0.020 |
| INR b | 2.83 ± 2.65 | 2.25± 0.77 | 1.80 ±0.57 | 0.015 |
| Aspartate transaminase; IU/L b | 275.14 ± 229.67 | 223.81 ±124.70 | 200.08 ± 53.77 | 0.932 |
| Alanine transaminase; IU/L b | 133.81 ± 122.01 | 115.21 ± 68.16 | 116.52 ± 40.14 | 0.614 |
| Alkaline phosphatase; IU/L b | 268.03 ±109.86 | 233.35 ±76.90 | 251.12 ± 93.78 | 0.421 |
| Serum albumin; g/dl b | 2.41 ± 0.41 | 2.28 ± 0.40 | 2.51 ± 0.41 | 0.089 |
| Serum protein; g/dl b | 6.23 ± 0.99 | 5.62 ±1.02 | 6.11 ± 0.63 | 0.003 |
| Total bilirubin; mg/dL b | 4.05 ± 1.69 | 3.68 ± 2.18 | 3.02 ± 1.11 | 0.027 |
| Direct bilirubin; mg/dL b | 2.45 ±1.32 | 2.20 ± 1.29 | 1.80 ± 0.85 | 0.136 |
| Blood urea nitrogen; mg/dL b | 44.36 ± 22.04 | 23.23 ± 14.80 | 25.20 ±12.94 | <0.001 |
| Creatinine; mg/dL b | 1.50 ± 0.51 | 1.47 ± 0.89 | 1.39 ± 0.71 | 0.071 |
| Ascitic fluid albumin; g/dL b | 0.61 ± 0.31 | 0.66 ± 0.36 | 0.87 ± 0.27 | 0.005 |
| Ascitic fluid protein; g/dL b | 1.01 ± 0.49 | 1.23± 0.51 | 1.12 ± 0.35 | 0.026 |
| Ascitic fluid LDH; SU b | 160.84 ± 83.17 | 166.95 ± 96.36 | 146.80 ± 67.69 | 0.479 |
| SAAG b | 1.80 ± 0.46 | 1.62 ± 0.49 | 1.64 ± 0.39 | 0.254 |
| SAAG d | 0.132 | |||
| Low | 3 (4.7) | 7 (16.3) | 3 (12.0) | |
| High | 61 (95.3) | 36 (83.7) | 22 (88.0) | |
| MELD score b | 24.20 ± 7.06 | 21.72 ± 6.86 | 19.24 ± 5.52 | <0.001 |
| Culture d | <0.001 | |||
| Escherichia coli | 40 (62.5) | 0 (0) | 10 (40.0) | |
| Staphylococci | 17 (26.5) | 0 (0) | 12 (48.0) | |
| Enterobacter | 7 (10.9) | 0 (0) | 3 (12.0) |
Abbreviations: SBP, spontaneous bacterial peritonitis; BA, bacterascites; CNNA, culture negative neutrocytic ascites; PTT, partial thromboplastin time; INR, international normalized ratio; LDH, lactic dehydrogenase; SAAG, serum-ascites albumin gradient; MELD, model for end-stage liver disease.
a Values are expressed as No. (%) or mean ± SD.
b Kruskal Wallis test.
c One-way ANOVA.
d Chi-square test.
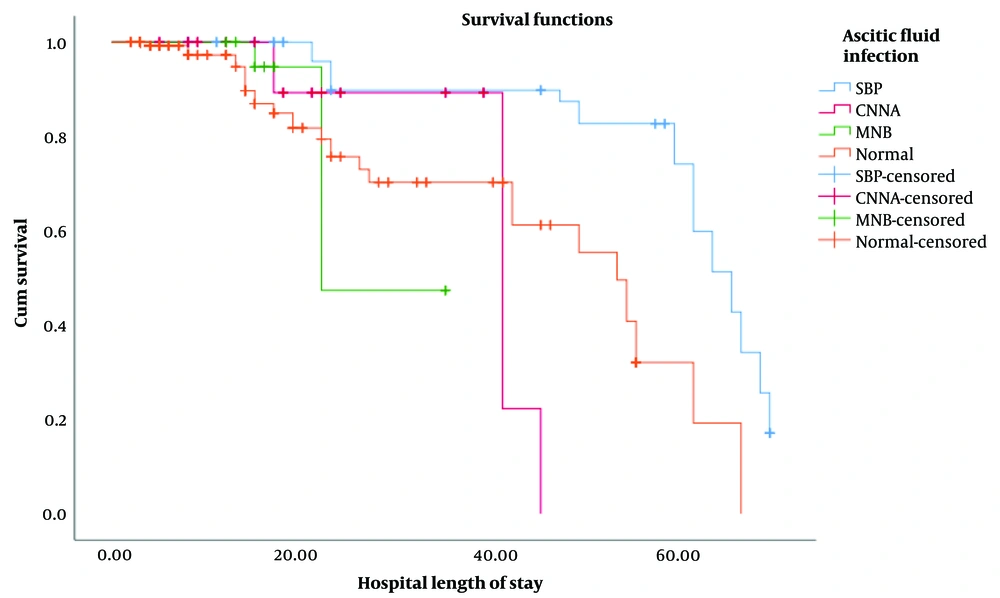
For patients with SBP, CNNA, BA, and the non-AFI group, the median/mean (SD) hospital length of stay (LOS) was 57.50/45.33 (22.53), 21.00/21.14 (11.45), 16.00/18.12 (6.95), and 6.00/13.68 (15.32) days, respectively. In the survival analysis, the Kaplan-Meier curve (Figure 1) and log-rank test demonstrated that the probability of death at any given time in SBP patients was significantly higher than in CNNA (P = 0.001), BA (P = 0.001), and non-AFI (P < 0.001) groups. However, there was no significant difference in the log-rank test between other groups, including CNNA, BA, and non-AFI participants (Figure 1).
Kaplan-Meier chart showing the probability of death at any time for patients with ascitic fluid infection variants (n : 132) versus non-infected ascites (n: 334). Abbreviations: SBP, spontaneous bacterial peritonitis; CNNA, culture negative neutrocytic ascites; MNB, mono bacterial non-neutrocyticbacter ascites.
Cox regression analysis (Table 4) showed that SBP (HR 2.43; 95% CI 1.36 - 4.36; P = 0.003) significantly increased the risk of mortality, while CNNA (HR 1.10; 95% CI 0.43 - 2.84; P = 0.84) and BA (HR 1.52; 95% CI 0.52 - 4.50; P = 0.45) did not significantly increase this risk. The presence of hepatic encephalopathy (HR 2.12; 95% CI 1.26 - 3.56; P = 0.005) and female gender also significantly increased the risk of mortality.
| Variables | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Gender | < 0.001 | < 0.001 | ||
| Female | 3.58 (2.22 - 5.79) | 2.51 (1.495 - 4.209) | ||
| Male | 1 | 1 | ||
| Age | 1.02 (1.00 - 1.03) | 0.04 | 1.02 (0.99 - 1.03) | 0.08 |
| SBP | 3.51 (2.08 - 5.93) | < 0.001 | 2.43 (1.36 - 4.36) | < 0.01 |
| CNNA | 0.81 (0.36 - 1.82) | 0.62 | 1.10 (0.43 - 2.84) | 0.84 |
| BA | 0.59 (0.21 - 1.68) | 0.32 | 1.52 (0.51 - 4.50) | 0.45 |
| Hepatic encephalopathy | 2.54 (1.48 - 4.36) | < 0.01 | 2.12 (1.26 - 3.56) | < 0.01 |
| Gastrointestinal bleeding | 1.12 (0.70 - 1.80) | 0.64 | 1.67 (0.97 - 2.86) | 0.07 |
| Renal failure | 0.90 (0.59 - 1.38) | 0.62 | 1.21 (0.73 - 2.02) | 0.45 |
| MELD score | 0.95 (0.918 - 0.98) | < 0.01 | 0.97 (0.94 - 1.01) | 0.11 |
Abbreviations: SBP, spontaneous bacterial peritonitis; BA, bacterascites; CNNA, culture negative neutrocytic ascites; MELD, model for end-stage liver disease.
5. Discussion
This is the first report from Iran to compare the outcomes, clinical features, and laboratory parameters among the three variants of AFI in patients with cirrhosis. The study found that SBP significantly increased the risk of mortality, while CNNA and BA did not significantly increase that risk. Furthermore, there were significant differences in clinical features and laboratory parameters, such as abdominal pain, peripheral edema, fever, nausea/vomiting, hepatic encephalopathy, renal failure, hemoglobin, INR, bilirubin, BUN, MELD score, ascitic fluid protein, and albumin, among the three AFI groups.
Ascitic fluid infection is the most common bacterial infection in patients with cirrhosis and is divided into different variants (1-3). The incidence of SBP, a typical variant of AFI, varies in studies, but it has been reported in up to 30% of cirrhotic patients with ascites. Although bacterial translocation from the gut plays a central role, changes in gut microbiota, intestinal permeability, and immune system function may also contribute to the progression of SBP. The classic SBP presentation includes fever, abdominal pain, and worsening of ascites. However, the diagnosis of SBP and other infections may be challenging, as classic symptoms are often absent, and a high index of suspicion is usually required for early diagnosis and treatment (2, 3).
Bacterascites is another variant of AFI, and its prevalence is about 10% of patients with cirrhosis and ascites. The clinical significance of BA varies depending on how the infection is acquired (1, 4). Culture-negative neutrocytic ascites is another variant of AFI (5) whose exact prevalence and outcome are still unknown (6-9).
Previous studies have reported different results on the outcomes and clinical manifestations of different types of AFI. In a study by Pelletier et al. (11) in 38 SBP patients and 15 CNNA participants, there was no difference in clinical signs and symptoms, but the mortality rate in patients with SBP was significantly higher than in patients with CNNA, which is consistent with the findings of our study.
A study conducted by Kim et al. (8) compared the clinical features and prognosis of CNNA and SBP in 130 hospitalized patients with cirrhosis and hepatitis B. Among these patients, 71.5% had CNNA and 28.5% had SBP. Similar to our results, patients with SBP showed higher in-hospital mortality than participants with CNNA. Based on logistic regression analysis, they showed that positive ascitic fluid culture was the only independent predictor of mortality in the hospital, but in our participants, female gender and hepatic encephalopathy, in addition to SBP, also significantly increased the risk of mortality.
A retrospective study at a hospital in China conducted by Ning et al. on 408 patients with SBP and 192 participants with BA found that, similar to our results, patients with BA had a lower mortality rate than those with SBP (4). In another prospective study, Runyon compared 44 episodes of monomicrobial non-neutrocytic bacterascites to 94 episodes of SBP and concluded that the mortality rate was similar in the two groups, which was inconsistent with our results (10).
Gram-negative bacteria, including Escherichia coli and Klebsiella spp., are the main causes of SBP. On the other hand, the most common gram-positive bacteria are Streptococcus spp., Enterococci spp., and Staphylococci spp. (2). In our study, the most common bacteria causing AFI were Escherichia coli, followed by Staphylococcus and Enterobacter. A study in India aimed at identifying the prevalence of various organisms causing SBP found Escherichia coli to be the most common pathogen, similar to our study (12). In another report conducted by Bibi et al., Escherichia coli (65%) was the predominant pathogen, followed by Enterococcus species (15%) (13). In a retrospective study by Oey et al., 123 patients with BA and SBP were studied, and Staphylococcus and Streptococcus were the most common microorganisms. The rate of cumulative mortality in BA patients was statistically comparable to that of SBP participants. They concluded that patients with BA and SBP were very comparable in overall prognosis and severity of liver disease (1). The findings of this research were entirely different from the results of our study.
Previous studies have compared the outcomes of SBP with CNNA, but the results are heterogeneous. In our study, SBP significantly increased the risk of mortality compared to CNNA. Srivastava et al. conducted a study in children with chronic liver disease to evaluate the clinical features and outcomes of various types of AFI. Similar to our study, they concluded that in-hospital mortality was higher in patients with SBP than in CNNA participants (7). In another study by Kamani et al., data from 44 patients with SBP and 143 participants with CNNA were analyzed. They concluded that patients with SBP had a higher mortality rate than those with CNNA, which was consistent with our results (6). A study by Na et al. compared the clinical characteristics and outcomes of 274 patients with CNNA and 259 participants with hospitalized SBP. They found that the seven-day mortality rate in SBP patients was higher than in CNNA patients, but the 30-day and 90-day mortality rates were similar in both groups (9). Terg et al. reported mortality rates of 36% and 46% in the first episode of SBP and CNNA, respectively. However, the probability of survival at 12 months was 32% in SBP and 31% in CNNA (14).
One strength of our study was the comparison of the three types of AFI with each other, as well as with the non-AFI group, considering many confounding factors. One important limitation was that we evaluated only in-hospital outcomes of AFI variants. Another limitation was that the AFI sample size in our study was relatively small. However, we selected a non-AFI group to compare with the AFI patients and optimally evaluated clinical features and laboratory parameter details in all AFI variants at the time of hospitalization to overcome this limitation. Finally, the study was conducted in one center, so a multicenter study is recommended.
5.1. Conclusions
Mortality risk was higher in patients with SBP than in those with other types of AFI. This study also showed differences in clinical characteristics and laboratory parameters among the three types of AFI. Further research is recommended to compare these variants of AFI more comprehensively.