1. Context
The emergence and spread of antimicrobial resistance among microorganisms has become a major concern worldwide, making this topic crucial to investigate. The relationship between antibiotic overuse and misuse, virulence, and antibiotic resistance is complex. Multiple factors associated with bacteria and their environments influence the evolution of both antibiotic resistance and virulence (1).
Overuse and misuse of antibiotics are major contributors to the development of resistance mechanisms. When antibiotics are used inappropriately or unnecessarily, bacteria are exposed to these drugs more frequently. This exposure creates selective pressure, favoring the survival and proliferation of resistant bacteria. The dissemination of antimicrobial resistance results in treatment failures, disease progression, and increased healthcare costs (2, 3).
Prior to conducting this study, it was well understood that resistant bacteria can become prevalent in populations, making infections harder to treat and leading to poor outcomes (4). Several factors contribute to the overuse and misuse of antibiotics, including inappropriate prescribing practices, patient demand for antibiotics even when not necessary, and the use of antibiotics in livestock and agriculture. In some cases, antibiotics are prescribed for viral infections, for which they have no effect, thereby contributing to the development of resistance without any benefit to the patient (5).
Addressing antibiotic resistance requires a multifaceted approach, including improving antibiotic stewardship practices, promoting appropriate antibiotic prescribing, and implementing infection prevention and control measures. Public education and awareness campaigns are also vital in helping individuals understand the importance of responsible antibiotic use and the consequences of antibiotic resistance (6).
Globally, efforts are underway to tackle antibiotic resistance through the development of new antibiotics, promoting research on alternative therapies, and implementing policies to regulate antibiotic use. It is crucial for healthcare providers, policymakers, and individuals to work together to combat antibiotic resistance and preserve the effectiveness of these life-saving medications (7).
In response to the rapid spread of antibiotic resistance and the lack of viable medications for treating infections caused by multi-drug resistant (MDR) bacteria in both human and animal medicine, scientists have been compelled to develop new antibacterial techniques. Natural products have historically been effective treatments for bacterial infections and continue to be a key source for developing new antibacterial medications (8).
There is a risk of entering a so-called "post-antibiotic era" in the near future, where infections that were once easily controlled could become lethal threats. Multidrug-resistant bacteria, which are defined as those that have developed resistance to at least three different classes of antimicrobials, have become widespread, especially in hospitals (9). Since the peak of the antibiotic era in the mid-20th century, natural compounds have served as effective therapies against pathogenic microorganisms. However, the rising incidence of antibiotic-resistant infections makes it clear that new solutions are needed (10).
Recent studies have reported the successful covalent attachment of polymeric antimicrobial materials onto surfaces such as glass, metal, paper, and polymers. Often, these biocidal polymers include cationic groups, such as alkyl pyridinium or quaternary ammonium. Cationic antimicrobials are particularly well-suited as self-disinfecting surfaces. For instance, polymer microspheres containing quaternized poly-2-(dimethylamino) ethyl methacrylate (poly DMAEMA) have demonstrated high levels of antibacterial activity. Within the scope of surface-active compounds, quaternary ammonium salts (QAS) as cationic antimicrobials show great promise (11). One of the most promising alternatives to antibiotics is the use of antimicrobial peptides, which can treat bacterial infections, particularly those caused by multidrug-resistant bacteria (12). Despite encouraging in vitro results, few formulations are currently used in clinical trials, partly due to the high cost of these drugs (13).
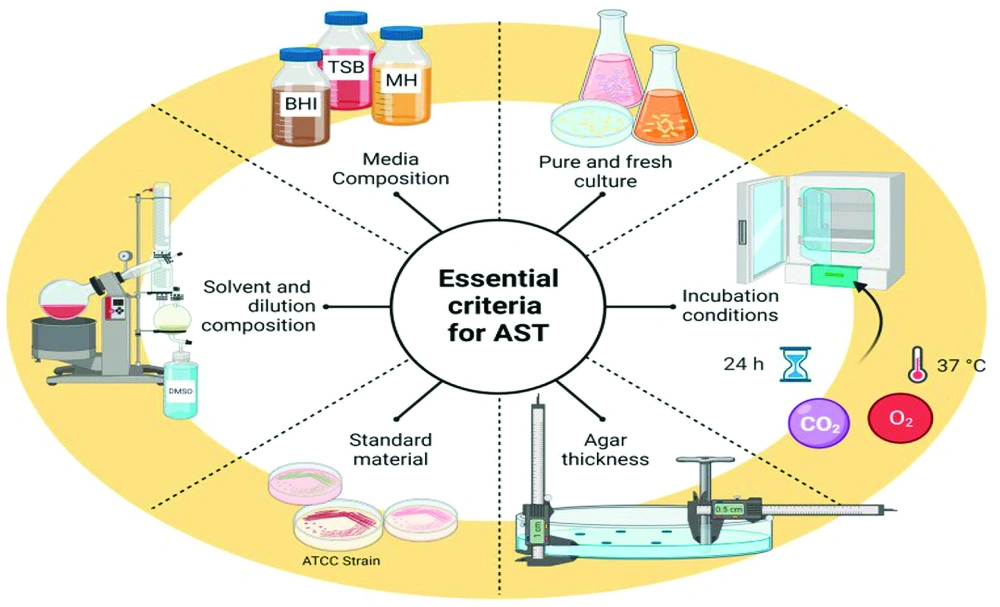
When libraries of crude extracts are screened, numerous bioactive molecules with effective antimicrobial properties can be identified. However, high rediscovery rates of previously known compounds linked to pre-existing resistance mechanisms, as well as a large number of hits with significant cytotoxicity or poor ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties, limit their potential for revealing novel solutions (14). Figure 1 illustrates key considerations for assessing antibacterial susceptibility tests (AST) in natural compounds.
2. Objectives
The goal of our research was to provide further insights into combating antibiotic resistance and addressing the urgent need for additional effective antimicrobial treatments as alternatives.
Key considerations to assess antibacterial susceptibility tests (AST) in natural compounds; Abbreviations: BHI, Bain heart infusion, TBS, tryptic soy broth, MH, Mueller Hinton, ATCC, American type culture collection, DMSO, dimethyl sulfoxide (15)
3. Methods
The search for relevant studies was determined based on molecular descriptors, which play a crucial role in understanding the physicochemical and biological properties of chemical compounds. Molecular descriptors provide quantitative representations of molecular structures, enabling the prediction and classification of various molecular properties. Articles were excluded based on specific criteria such as being unrelated to the topic, having an unknown plant or compound name, lacking a validated source of material, or employing inappropriate methodologies.
3.1. Evidence Acquisition
This narrative review is based on clinical trials and in vitro examination studies. The method involved reviewing various studies and findings that highlighted the antimicrobial properties of natural compounds and extracts. Additionally, the mechanisms of action exerted by these natural compounds and their antimicrobial activity were also considered.
3.2. Statistics
This review is not a systematic or structured narrative but rather a descriptive one. It presents the authors' subjective views, focusing on a general description of the study findings and providing a summary of the qualitative results.
4. Results
Natural extracts have long been used to treat a wide range of medical conditions, from infectious diseases to cancer, due to their convenience and therapeutic potential. Natural products derived from microbes, plants, and animals offer a broad variety of molecules and chemical compounds. They serve as one of the most important sources for innovative drug development for both animal and human health. Moreover, natural extracts inspire synthetic biology and chemistry scientists in the discovery of new bioactive compounds and pharmaceuticals (16).
Additionally, we highlighted the advantages of using natural compounds and extracts as antimicrobial agents. These advantages include their potential for multi-target activity, a lower likelihood of resistance development, and their alignment with sustainable and eco-friendly approaches to healthcare. Currently, a wide range of methodologies is employed to analyze the in vitro activity of natural extracts to determine their suitability as antimicrobial agents. While traditional technologies remain widely used, technological advances have led to the implementation of methods that address challenges related to analysis capacity, time, sensitivity, and reproducibility (17, 18).
Natural compounds and extracts that could potentially serve as solutions to the antibiotic resistance crisis include the following.
4.1. Antimicrobial Peptides (AMPs) and Cell-Penetrating Peptides (CPPs)
Antimicrobial peptides are short, cationic peptides known for their antibacterial action. Comprising 10 - 60 amino acid residues, AMPs can be synthetic or natural and are effective against bacteria, viruses, and fungi by destroying their cells. Antimicrobial Peptides are found in nearly all living organisms, from prokaryotes to more complex eukaryotes, and vary in molecular weight and amino acid residue counts, ranging from five to over 100. Most AMPs are amphiphilic and cationic, consisting of short sequences of positively charged amino acids. These peptides exhibit a broad range of antibacterial activity with remarkable specificity and minimal toxicity, making them a promising solution to combat antibiotic resistance (19).
Plants are a significant source of AMPs, which are produced as part of their self-defense mechanisms. Classes of AMPs found in plants include snakins, thionins, lipid transport proteins, glycine-rich proteins, defensins, cyclotides, and hevein-type proteins. These AMPs are distributed across different plant organs, including stems, roots, seeds, flowers, and leaves. They play crucial roles in the plant's defense by eliminating viruses, bacteria, and parasites, making them potential candidates for use as therapeutic and preservation agents (20). In humans, AMPs are synthesized by macrophages, granulocytes, and most epithelial cells, where they exhibit antiviral, antibacterial, antifungal, and antiprotozoal properties. Some AMPs have even progressed to clinical trials as novel treatments against antibiotic-resistant bacteria due to their potent bactericidal capabilities (21). Cell-penetrating peptides (CPPs), small compounds with a positive charge composed of 5 to 30 natural or synthetic amino acid residues, are another class of peptides. Cell-penetrating peptides can traverse various biological membranes and have been utilized as carriers for delivering diverse conjugated cargo. While CPPs enhance the transport of antibiotics by passing through the microbial envelope barrier, AMPs exert their antimicrobial activity by damaging bacterial membranes (22).
Researchers have synthesized thousands of AMPs with a wide spectrum of biological effects, targeting fungi, bacteria, protozoa, enveloped viruses, and even cancer cells. Beyond their direct antimicrobial effects, AMPs possess immunomodulatory functions, which are crucial for regulating the adaptive immune system and controlling inflammation (23). As potential alternatives to traditional antibiotics, AMPs offer various mechanisms for combating bacterial infections, including direct bactericidal action and immunomodulatory effects (24). Compared to empirical antibiotics, AMPs demonstrate several advantages, such as a lower tendency to select for resistance, rapid killing action, broad-spectrum activity, and exceptional clinical effectiveness against multiple MDR bacteria (25).
4.2. Nanoparticles
Recent research has indeed shown promising results regarding the antiviral and antibacterial properties of nanoparticles. Nanoparticles possess unique characteristics that make them highly effective in targeting and inhibiting the growth of viruses and bacteria. These particles can interact with microbial cells, disrupt their structure or function, and prevent their replication or spread (26).
One area of focus in nanoparticle research is the development of environmentally friendly production methods. Traditional synthesis techniques often involve the use of toxic chemicals or high-energy processes, which can have detrimental effects on the environment. To mitigate these issues, researchers are exploring the use of natural substances such as plant extracts, fruit juices, or other relevant sources to create nanoparticles in a more sustainable and eco-friendly manner (27, 28). The use of natural substances in nanoparticle production offers several advantages. These substances often contain bioactive compounds with inherent antimicrobial properties, which can enhance the effectiveness of the nanoparticles against viruses and bacteria. Additionally, natural sources are generally more readily available, cost-effective, and pose fewer risks to human health and the environment (29).
The antiviral and antibacterial properties of these nanoparticles are attributed to various mechanisms. For example, nanoparticles can physically interact with viral or bacterial surfaces, disrupting their membranes and preventing their entry into host cells. They can also inhibit viral replication or bacterial growth by interfering with essential cellular processes or by releasing antimicrobial agents (30, 31)). Moreover, nanoparticles can be engineered to specifically target certain viruses or bacteria, enhancing their efficacy and reducing potential side effects. Functionalization with ligands or antibodies that recognize specific microbial markers allows for precise and targeted delivery of nanoparticles to infected cells (32).
The potential applications of these nanoparticles in combating life-threatening viral and microbial diseases are extensive. They can be used in the development of antiviral drugs, disinfectants, or coatings for medical devices to prevent infections. Additionally, nanoparticles can be employed in water treatment or air purification systems to remove pathogens and improve public health (33, 34). However, it is important to note that research in this field is still ongoing. Further studies are necessary to fully understand the safety, efficacy, and long-term effects of these nanoparticles. Additionally, regulatory considerations and ethical implications must be addressed before widespread implementation can occur (35).
4.3. Enzyme Disruption of the Acyl Homoserine Lactones (AHLs)
Quorum quenching (QQ) is a mechanism employed by bacteria to disrupt communication and coordination within bacterial colonies. This process involves the enzymatic breakdown of signaling molecules called acylhomoserine lactones, which are used by bacteria for cell-to-cell communication, a process known as quorum sensing (36-38).
The quorum quenching acylases that act on N-acylhomoserine lactones belong to the Ntn hydrolase superfamily, which also includes β-lactam acylases such as penicillin G acylases (PGA), penicillin V acylases (PVA), and cephalosporin acylases (CA). Recent findings reveal structural similarities and significant overlaps in the substrate spectra of these enzymes, providing insights into possible links between bacterial signaling and antibiotic resistance (39).
Paraoxonases are one type of enzyme that can break down the acylated chain of AHLs, rendering them inactive. Acyl homoserine Lactones -acylases, on the other hand, catalyze the hydrolysis of the lactone ring present in AHLs, leading to the breakdown of these molecules and the loss of their signaling capabilities. Additionally, AHL lactonases, which belong to the metalloproteinase group, hydrolyze the ester bond of the HSL ring, forming acyl homoserine and rendering the AHLs non-functional (40). By cleaving or disrupting AHL signaling molecules, quorum quenching prevents bacteria from coordinating activities based on population density. This disruption can inhibit the production of virulence factors or biofilm formation, both of which are crucial for bacterial pathogenicity and survival (41). The application of quorum quenching and the use of enzymes involved in this process have gained attention in various fields. For example, in biotechnology, quorum quenching enzymes can be used to control bacterial infections or biofouling in industrial processes. In medicine, the modulation of quorum sensing through quorum quenching is being explored as a potential strategy to combat bacterial infections and enhance the effectiveness of antibiotics (40). It is important to note that research in this area is still ongoing, and the full potential and limitations of quorum quenching as a therapeutic or biotechnological tool are still being explored (42).
4.4. Mur Enzymes
Efforts are underway to identify and develop novel antibacterial drugs that target peptidoglycan synthesis, with particular focus on the Mur enzymes. Peptidoglycan (PG) synthesis is essential for bacterial survival and represents a critical target for the development of new antimicrobial drugs. Peptidoglycan, a major component of bacterial cell walls, plays a crucial role in maintaining cell shape, integrity, and protection against osmotic stress (43).
The synthesis of peptidoglycan involves a series of enzymatic steps and the utilization of various precursor molecules. Enzymes such as GlmS, GlmM, and GlmU are involved in the precursor biosynthesis pathway, leading to the formation of UDP-N-acetylglucosamine (GlcNAc) and UDP-N-acetylmuramic acid. These precursor molecules are then utilized by subsequent enzymes in the peptidoglycan synthesis pathway. Mur enzymes, including Mur ligases, MraY, and MurG, are involved in the later stages of peptidoglycan synthesis. Mur ligases catalyze the formation of peptide bridges between N-acetylmuramic acid and N-acetylglucosamine units, while MraY and MurG are responsible for the transfer of lipid intermediates and the final assembly of the peptidoglycan layer (44).
Targeting enzymes involved in peptidoglycan synthesis, such as the Mur enzymes, has been recognized as a promising strategy for developing antibacterial agents. Inhibiting these enzymes can disrupt peptidoglycan synthesis, leading to cell wall defects and bacterial death (45). The specificity of targeting peptidoglycan synthesis is advantageous because these enzymes have no human homologs. As a result, drugs designed to inhibit these enzymes are less likely to have off-target effects on human cells, enhancing their potential as selective antimicrobial agents (46).
However, the development of effective drugs targeting these enzymes remains a complex and ongoing area of research, as bacteria can develop resistance mechanisms against such drugs. Continued research and exploration of alternative targets and strategies are necessary to combat the growing challenge of bacterial resistance and to develop effective antimicrobial therapies (47).
4.5. Lysocine E
Lysocin E is a natural product that has been identified as a specific inhibitor of menaquinone, a key component of bacterial cell membranes. This compound has demonstrated higher efficacy compared to vancomycin, a commonly used antibiotic. Menaquinones are essential for the structure and function of cell membranes in various bacteria, including anaerobic bacteria, Mycobacteria, and Gram-positive bacteria. They play crucial roles in processes such as the production of endospores, cytochromes, and the electron transport chain.
By specifically targeting menaquinone, lysocin E disrupts the integrity and function of bacterial cell membranes. This disruption impairs vital cellular processes, ultimately leading to bacterial cell death. The higher efficacy of lysocin E compared to vancomycin suggests its potential as a promising alternative or adjunctive therapy for treating bacterial infections. However, further research is necessary to fully explore the therapeutic potential of lysocin E and its efficacy against different types of bacteria.
The biosynthesis of menaquinones involves several enzymes, including MenH, MenD, MenF, MenG, MenC, MenB, MenA, and MenE. Inhibitors of these enzymes have been identified, with many being analogs of the enzyme's cofactors or substrates. By targeting these bacterial-specific enzymes, researchers can develop new antimicrobial drugs to combat bacterial infections (48).
In addition to synthetic inhibitors, natural products derived from plants have also shown significant medicinal value in treating human diseases. Medicinal plants have been extensively studied for their active ingredients and have served as a source of natural compounds with potential therapeutic properties. In the context of antibiotic resistance, in vitro antibacterial or antifungal assays are commonly used to evaluate the efficacy of plant-derived natural products against bacterial and fungal infections (49-52).
4.6. Plant-Derived Natural Products
Botanical medicine, or the use of plant-derived compounds for medicinal purposes, has a long history as a source of antimicrobial agents due to the rich concentration of active ingredients with medicinal properties found in plants. These natural compounds can act as antimicrobial agents through various mechanisms, such as disrupting cell membranes, inhibiting enzyme activity, interfering with microbial DNA replication, or modulating the immune system. In vitro tests have demonstrated that many plant-derived natural compounds possess antibacterial and anti-biofilm properties. These bioactive compounds, derived from botanical sources or medicinal plants, have shown the ability to inhibit the formation of biofilms, which are protective layers that bacteria form to shield themselves from antibiotics and the immune system (53).
Medicinal plants have been extensively studied as a potential source for various compounds used in the treatment of human diseases. With antibiotic resistance becoming a global concern, it is crucial to evaluate the potential of plant-derived natural products in combating bacterial and fungal infections. These natural products can provide alternative options for the development of new antimicrobial agents. The initial step in assessing the significance of medicinal plants is conducting in vitro antibacterial or antifungal assays. In these assays, researchers test the effectiveness of plant extracts or isolated compounds against specific bacteria or fungi in a controlled laboratory setting. By conducting these assays, researchers can determine the antimicrobial activity of plant-derived natural products. This helps in identifying potential candidates for further development as alternative or complementary treatments to combat antibiotic-resistant infections.
Further research and development are necessary to understand the mechanisms of action, optimize formulations, and evaluate the safety and efficacy of these plant-derived compounds. Nonetheless, exploring the potential of medicinal plants in the search for new antimicrobial agents is a promising approach to address the challenge of antibiotic resistance (52). Some notable examples of botanical antimicrobial agents include: (1) tea tree (melaleucaalternifolia) oil: Derived from the leaves of the tea tree, tea tree oil has broad-spectrum antimicrobial activity against bacteria, fungi, and viruses. It is commonly used topically for skin infections and is also found in oral care products; (2) echinacea (echinaceapurpurea): Echinacea extracts, derived from the purple coneflower, have been traditionally used to boost the immune system and fight respiratory infections. They have shown antimicrobial activity against various bacteria and viruses; (3) turmeric (curcuma longa and curcuma aromatica): The active compound in turmeric, curcumin, has been found to possess antimicrobial properties. It has shown efficacy against a wide range of bacteria, including antibiotic-resistant strains; (4) garlic (alliumsativum): Garlic contains several sulfur compounds, such as allicin, that have potent antimicrobial properties. Garlic has been used for centuries to treat various infections, including respiratory and gastrointestinal infections; (5) cranberry (vacciniummacrocarpon): Cranberry extracts have been studied for their ability to prevent urinary tract infections (UTIs) by inhibiting the adhesion of bacteria, such as Escherichia coli, to the urinary tract lining (53-55). These examples illustrate the diverse potential of botanical medicine in addressing the growing threat of antibiotic resistance. By leveraging the natural antimicrobial properties of these and other plants, researchers can develop new strategies to combat infections, particularly those caused by antibiotic-resistant pathogens.
4.7. Polyphenols
Polyphenols are a class of naturally occurring compounds widely found in fruits, vegetables, nuts, seeds, herbs, and beverages such as tea and coffee. They are well-known for their antioxidant properties and have been extensively studied for their potential health benefits. Polyphenols are characterized by their chemical structure, which includes multiple phenol rings. They can be further classified into different subclasses, including flavonoids (such as anthocyanins, flavones, flavonols, and isoflavones), phenolic acids (such as caffeic acid and gallic acid), stilbenes (such as resveratrol), and lignans (56).
One of the significant health benefits associated with polyphenols is their potential antimicrobial activity. Numerous studies have demonstrated the ability of polyphenols to inhibit the growth of various bacteria, fungi, and viruses. These compounds can act by disrupting microbial cell membranes, inhibiting enzyme activity, or interfering with microbial DNA replication (57).
Polyphenols have shown antimicrobial activity against a wide range of microorganisms, including antibiotic-resistant strains. They have been investigated for their potential in treating and preventing various infections, including respiratory tract infections, gastrointestinal infections, urinary tract infections, and skin infections. In addition to their antimicrobial properties, polyphenols have been studied for their antioxidant, anti-inflammatory, and immune-modulating effects. They have also been associated with potential benefits in cardiovascular health, cancer prevention, neuroprotection, and metabolic disorders.
It is important to note that the bioavailability and effectiveness of polyphenols can vary depending on factors such as the food source, processing methods, and individual variations in metabolism. Further research is needed to better understand the mechanisms of action and optimal dosages of polyphenols for specific health conditions (58).
Incorporating a variety of polyphenol-rich foods into a balanced diet is generally recommended to benefit from their potential health-promoting effects. However, it is always advisable to consult with healthcare professionals for personalized advice and guidance (59).
4.8. Nanozymes
Nanoparticles (NPs), often referred to as invisible particulate substances with diameters ranging from 1 - 100 nm, have become highly relevant in modern science (60, 61). Their large surface area to volume ratio makes them unique as delivery systems and antimicrobial agents in many respects. Over the past few decades, nanoparticles have been utilized for human welfare across various fields, including medical science, drug delivery, electronics, optics, agriculture, wastewater treatment, and sensor support (62, 63).
Recently, there has been intense scientific exploration of nanoparticles due to their potential applications in diverse areas. In particular, nanozymes—a new class of antibiotics—have emerged with promising features such as broad-spectrum antibacterial capabilities and low toxicity. Nanoparticles are considered potential antimicrobial agents due to their affinity for sulfur-rich amino acids, their ability to adhere to microbial cell walls via electrostatic attraction, and their capacity to disrupt microbial cytoplasmic membranes and nucleic acids. However, despite their potential, nanoparticles have certain limitations that can affect their antibacterial efficacy (64).
One notable limitation is their reduced enzymatic activity compared to natural enzymes. Nanozymes may not possess the same level of catalytic efficiency as natural enzymes, which can impact their ability to effectively target and eliminate bacteria. Another limitation is their inability to trap or immobilize bacteria, which can hinder their effectiveness in physically capturing and holding onto microbes. These limitations can result in inferior antibacterial efficacy compared to traditional antibiotics. However, it is important to note that nanozymes are still an emerging field of research, and ongoing efforts are being made to overcome these challenges. Scientists are actively working on enhancing the enzymatic activity of nanozymes and developing strategies to improve their ability to trap and eliminate bacteria. With further advancements and refinement, nanozymes have the potential to become powerful tools in combating bacterial infections, particularly in the context of antibiotic resistance (65).
4.9. Peptidomimetic
Currently, a new class of antimicrobial peptides (AMPs) termed "peptidomimetics" has been developed. These compounds can mimic the bactericidal mechanisms of AMPs while being resistant to enzymatic degradation, making them highly effective against multidrug-resistant bacteria. Peptidomimetics with alkyl substituents synthesized from amino acids have shown broad-spectrum antibacterial properties and hold significant potential in combating drug-resistant bacteria (66).
One notable example of this class is Murepavadin, a pathogen-specific antimicrobial peptidomimetic with a novel, non-lytic mechanism of action. It is the first in a class of antibiotics targeting outer membrane proteins, specifically developed to combat drug-resistant bacteria. Biochemical and genetic analyses revealed that Murepavadin targets a homolog of the beta-barrel protein LptD (Imp/OstA), which plays a critical role in outer membrane biogenesis. This peptidomimetic demonstrated potent antibacterial activity in a mouse infection model simulating septicemia, particularly against drug-resistant Pseudomonas bacteria—a significant public health concern (67). Murepavadin operates through a unique mechanism, preventing lipopolysaccharide transport to the outer membrane by inhibiting LptD. This action disrupts the integrity of the bacterial outer membrane, leading to bacterial death. Murepavadin has shown effectiveness against drug-resistant Pseudomonas aeruginosa, a notoriously difficult bacterium to treat, especially in individuals with cystic fibrosis. The development of Murepavadin is particularly encouraging given the limited number of compounds currently in Phase II clinical trials for treating multidrug-resistant Gram-negative pathogens. Notably, four out of the nine compounds in these trials belong to new classes of antibiotics. This is a promising development, considering the historically high failure rate of compounds in clinical trials (68). The emergence of these new antibiotic classes highlights the potential for expanding the arsenal of effective treatments against multidrug-resistant bacteria, particularly Gram-negative pathogens.
Further research and development are crucial to fully realize the therapeutic potential of these new antibiotics and to address the growing challenge of drug-resistant bacteria.
4.10. Antimicrobial Compounds with Fungi Origin
Fungi are a diverse group of microorganisms that have been the source of many antimicrobial compounds. They emerge as a promising source of such compounds because they produce a wide range of secondary metabolites with bacteriostatic or fungistatic activity. These compounds can serve as alternatives to commonly used antibiotics. Additionally, fungi also accumulate compounds with antiviral activity. Selected compounds of fungal origin belong to groups such as isoprenoids, peptides, nucleosides, and acetylene derivatives (69).
These compounds, produced by fungi themselves, are known as fungal secondary metabolites. Fungi have evolved these compounds as a defense mechanism against other microorganisms and to compete for resources in their environment. Here are some examples of antimicrobial compounds derived from fungi.
4.10.1. Penicillin
Penicillin and its derivatives, known as beta-lactam antibiotics, are highly effective against a wide range of bacteria. They work by interfering with the synthesis of bacterial cell walls, leading to the weakening and eventual lysis of the bacterial cells. This mechanism of action makes them particularly effective against Gram-positive bacteria, but they also exhibit activity against some Gram-negative bacteria. The discovery and development of penicillin paved the way for numerous other antibiotics and has been instrumental in treating various bacterial infections, including pneumonia, Streptococcal sore throat, skin infections, and urinary tract infections.
However, it is important to note that the overuse and misuse of antibiotics have led to the emergence of antibiotic-resistant bacteria. This has created an urgent need for the discovery and development of new antimicrobial agents to combat these resistant strains. Despite these challenges, penicillin remains a critically important drug in the treatment of bacterial infections and continues to save countless lives worldwide (70, 71).
4.10.2. Griseofulvin
Griseofulvin is an antifungal medication derived from the Penicillium griseofulvum fungus. First isolated in 1939, griseofulvin has been used for many years to treat various fungal infections, particularly those affecting the skin, hair, and nails. Griseofulvin works by inhibiting fungal cell division and growth. It binds to the protein keratin in the skin, hair, and nails, making these tissues resistant to fungal invasion. By disrupting the growth and reproduction of fungi, griseofulvin helps to eliminate the infection.
This medication is primarily used to treat dermatophyte infections, which are fungal infections that commonly affect the skin, hair, and nails. It is effective against various dermatophyte species, including Trichophyton, Microsporum, and Epidermophyton. Griseofulvin is typically taken orally, and its absorption is enhanced when taken with fatty foods. The treatment duration can vary depending on the type and severity of the infection, often lasting for several weeks to months. It's important to note that griseofulvin may have side effects, including gastrointestinal upset, headache, dizziness, and sensitivity to sunlight (72-74).
4.10.3. Amphotericin B
Amphotericin B is a potent antifungal medication derived from Streptomyces nodosus. It has been used for many years to treat serious and systemic fungal infections, particularly those caused by fungi resistant to other antifungal agents. Amphotericin B works by binding to a component of the fungal cell membrane called ergosterol. This binding disrupts the integrity and function of the fungal cell membrane, leading to leakage of intracellular contents and ultimately causing the death of the fungal cell.
Amphotericin B is primarily used to treat severe systemic fungal infections, such as invasive candidiasis, aspergillosis, cryptococcosis, and mucormycosis. It is also used in cases where other antifungal medications have proven ineffective or when the infection is caused by a resistant fungus. Due to its poor oral absorption, amphotericin B is typically administered intravenously, often in a hospital setting under close medical supervision. This is necessary because it can cause significant side effects, including kidney toxicity. Regular monitoring of kidney function and electrolyte levels is essential during treatment with amphotericin B.
Because of its potential side effects, amphotericin B is reserved for severe and life-threatening fungal infections. However, newer formulations and lipid-based preparations of amphotericin B have been developed to improve tolerability and reduce the risk of side effects (75).
4.10.4. Echinocandins
Echinocandins are a class of antifungal medications that target the cell wall of fungi. They are derived from various fungal species, including species of Aspergillus and Trichoderma. Echinocandins work by inhibiting the synthesis of beta-glucan, a crucial component of the fungal cell wall, thereby compromising the structural integrity of the fungus and leading to cell death.
The three main echinocandin medications currently available are caspofungin, micafungin, and anidulafungin. These medications are primarily used to treat invasive fungal infections, including candidemia (fungal bloodstream infections), invasive candidiasis, and invasive aspergillosis. Echinocandins are administered intravenously and are typically given in a hospital setting due to the severity of the infections they treat. They have a broad spectrum of activity against various fungal species, including Candida and Aspergillus species, making them effective against both yeast and mold infections.
Compared to other antifungal classes, echinocandins generally have a favorable safety profile with fewer drug interactions and lower toxicity. However, they may still cause some side effects, including gastrointestinal upset, headache, and changes in liver function tests. Echinocandins are considered a first-line treatment option for many invasive fungal infections, especially in critically ill patients or those with compromised immune systems. Healthcare providers determine the most appropriate echinocandin and dosage based on the specific fungal infection being treated and individual patient factors (76).
4.10.5. Statins
Statins are a class of medications used to lower cholesterol levels in the blood. They work by inhibiting the enzyme HMG-CoA reductase, which plays a key role in cholesterol synthesis in the liver. The enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase catalyzes the conversion of HMG-CoA to mevalonate, a critical step in the synthesis of cholesterol and other isoprenoids. By reducing cholesterol production, statins help to lower LDL cholesterol (often referred to as "bad" cholesterol) levels and decrease the risk of cardiovascular disease (77).
Statins are commonly prescribed for individuals with high cholesterol levels, particularly those with a history of heart disease, stroke, or other cardiovascular conditions. They are also prescribed as a preventive measure for individuals at high risk of developing cardiovascular disease, such as patients with diabetes or certain genetic conditions. In addition to lowering LDL cholesterol, statins have been shown to have other beneficial effects. They can increase HDL cholesterol (often referred to as "good" cholesterol) levels, reduce inflammation, stabilize plaques in blood vessels, and improve endothelial function. Commonly prescribed statins include atorvastatin, simvastatin, rosuvastatin, and pravastatin. These medications are typically taken orally and are available in various strengths.
While statins are generally well-tolerated, they can have side effects. The most common side effects include muscle pain or weakness (myalgia), liver enzyme abnormalities, and gastrointestinal symptoms. Rarely, statins can cause more serious side effects such as muscle breakdown (rhabdomyolysis) or liver damage, although these occurrences are rare (78).
It is important to note that statins are usually prescribed as part of a comprehensive approach to managing cholesterol levels and reducing cardiovascular risk. This includes lifestyle modifications such as a healthy diet, regular exercise, and smoking cessation. Regular monitoring of cholesterol levels and liver function is typically recommended during statin therapy (79).
4.10.6. Lactoferrin
Lactoferrin is a multifunctional glycoprotein naturally found in various bodily fluids, including milk, tears, and saliva. It plays a vital role in the innate immune system and offers several potential health benefits. One of the primary functions of lactoferrin is its ability to bind and transport iron. With its high affinity for iron, lactoferrin limits the availability of iron to bacteria and other pathogens, thereby inhibiting their growth and proliferation.
Lactoferrin also exhibits antimicrobial properties. It can directly kill or inhibit the growth of bacteria, viruses, and fungi. This antimicrobial effect is achieved by disrupting the integrity of microbial cell membranes, interfering with the replication and transcription of microbial DNA, and modulating immune responses (80).
In addition to its antimicrobial activity, lactoferrin has been found to have anti-inflammatory and immunomodulatory effects. It helps regulate immune responses, promotes wound healing, and modulates the gut microbiota. Due to its various biological activities, lactoferrin has been the subject of research investigating its potential therapeutic applications, such as promoting gastrointestinal health, enhancing immune function, preventing and treating infections, and even serving as an adjunct treatment for certain types of cancer.
Lactoferrin is available as a dietary supplement and is also added to some infant formulas and functional foods. However, it is important to note that research on lactoferrin is still ongoing, and its specific therapeutic applications and optimal dosages are not yet fully established (81-83).
These ongoing studies underscore the importance of lactoferrin and similar compounds in the development of new antimicrobial agents, particularly in the fight against drug-resistant microorganisms.
5. Conclusions
Antimicrobial resistance presents a pressing challenge to public health, necessitating the search for novel antimicrobial agents. While research in this area is promising, addressing antibiotic resistance requires a multifaceted approach. This includes promoting responsible antibiotic use, developing new antibiotics, and implementing robust infection prevention and control measures. Additionally, integrating alternative treatments, such as those derived from natural compounds and innovative therapies, offers a complementary strategy to combat specific medical conditions. The collective data and observations underscore the importance of a comprehensive and coordinated effort to mitigate the impact of antibiotic resistance and safeguard public health.