1. Background
Hydrocephalus, characterized by an abnormal accumulation of cerebrospinal fluid (CSF) within the brain, affects a significant number of individuals, necessitating advanced medical interventions for its management. Ventriculoperitoneal (VP) shunting is a widely employed and effective method to address hydrocephalus, alleviating symptoms and improving neurological outcomes (1, 2). In the United States alone, hydrocephalus leads to approximately 70,000 hospitalizations annually (3). With around 40,000 CSF shunts placed each year, the associated healthcare costs escalate into billions of dollars, underscoring the magnitude of the issue (4-6).
The efficacy of VP shunting in enhancing neurological function and reducing mortality and morbidity in hydrocephalic patients is noteworthy. However, the management of these shunts throughout a patient’s life is fraught with challenges. The potential for malfunction or infection necessitates vigilant monitoring and often requires surgical intervention or replacement. A VP shunt infection is defined as an instance where an organism is isolated from the CSF culture of the shunt or when specific symptoms and signs manifest in the patient, depending on their age (7). Despite the clear benefits of VP shunting, patients with hydrocephalus frequently undergo multiple revisions, with shunt dysfunction occurring in approximately 25% - 35% of patients within the first year (8, 9). Additionally, 70% - 80% of patients require one or more interventions, including re-shunt surgery, during their lifetime (10).
Shunt infections emerge as a critical complication, significantly impacting patients’ well-being. These infections not only elevate the risk of seizures and diminish mental function but also contribute to a two-fold increase in long-term mortality and neurological disability (11). Moreover, shunt infections extend hospital stays, adding to the economic burden on healthcare resources. Various risk factors are associated with VP shunt infection, including patient demographics such as age at initial shunt placement, hydrocephalus etiology, and intraoperative variables. However, the significance of these factors in infection development varies across studies (12-14). Notably, research focusing on pediatric cases is limited and lacks extensive statistical representation.
To address this gap, a case series study was conducted. This investigation delves into cases of VP shunt infection in children aged 0 to 16 years. The study focuses on patients admitted to the children’s department and neurosurgery special care department of Loghman Hakim Hospital from 2018 to September 2023. This timeframe provides a crucial period for understanding the epidemiology of infected VP shunts in pediatric hydrocephalic patients, contributing valuable insights to the existing body of knowledge.
2. Methods
2.1. Study Design and Population
Children with hydrocephalus hospitalized at Loghman Hakim Hospital between 2018 and September 2023 were enrolled in this study. The study centers on pediatric patients with hydrocephalus who received care in the hospital’s children’s department and special care neurosurgery department during the specified period. Hydrocephalus, characterized by abnormal CSF accumulation, is the primary medical condition of interest.
This study adopts a descriptive approach, aiming to comprehensively characterize the epidemiology of VP shunt infections in children with hydrocephalus. The focus is on understanding the incidence, patterns, and associated factors of these infections among patients hospitalized in the children’s department and special care neurosurgery department of Loghman Hakim Hospital from 2018 to September 2023.
2.2. Data Collection
The data for this research was obtained from patient files in the archive unit of Loghman Hospital.
2.3. Terms Definition
Ventriculoperitoneal shunt infection was defined as the presence of at least one of the following criteria: (1) isolation of an organism from one or more CSF cultures, or (2) the presence of fever (temperature > 38°C), headache, neck stiffness, cranial nerve signs, or irritability without another recognized cause (15).
2.4. Measurement and Tools
The information collection process in this study relies on observational methods, with a focus on meticulously examining medical records and relevant files to identify cases of VP shunt infections. This observational approach enables a thorough exploration of patient histories, ensuring accurate data collection. The process involves the following steps: (1) initial Identification, relevant cases are identified by extracting files labeled with VP shunt infection codes; (2) file Examination, the extracted files are carefully reviewed to confirm VP shunt infections based on the established criteria; (3) data collection, for each confirmed case of shunt infection, information forms are completed based on the research variables.
This study provides a comprehensive overview of the epidemiology of VP shunt infections in children with hydrocephalus. By employing a census method, the study ensures that data from all eligible cases admitted to the children’s department and special care neurosurgery department of Loghman Hakim Hospital between 2018 and September 2023 are included. The selection process involves extracting files labeled with VP shunt infection codes, confirming infections through file examination, and completing detailed information forms for each case.
2.5. Statistical Analyses
The data analysis was conducted using descriptive statistics to summarize demographic characteristics and pertinent variables with SPSS version 21. Frequency and percentage were used to describe categorical variables, while mean and standard deviation were used to characterize continuous variables. Due to the non-normal distribution of the data, the Mann–Whitney U test was applied for analyzing quantitative variables, and the chi-square test was used for qualitative variables. A P-value of less than 0.05 was considered statistically significant.
3. Results
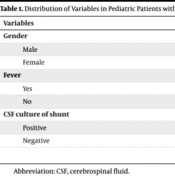
This investigation scrutinized the records of 39 pediatric patients with VP shunts admitted to Loghman Hakim Hospital between 2018 and 2023, with ages ranging from 1 month to 16 years. Among these 39 cases, 19 were male and 20 were female (Table 1).
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 19 (48.7) |
| Female | 20 (51.3) |
| Fever | |
| Yes | 9 (75) |
| No | 3 (25) |
| CSF culture of shunt | |
| Positive | 5 (41.7) |
| Negative | 7 (58.3) |
Abbreviation: CSF, cerebrospinal fluid.
Among the 39 cases with VP shunts due to hydrocephalus, the etiologies were diverse: 18 cases were associated with brain tumors, 14 cases with structural brain disorders, 2 cases with meningitis, 2 cases with meningomyelocele, and 2 cases with head trauma. A single case was attributed to coagulation factor deficiency. Notably, the most prevalent brain tumor leading to hydrocephalus was identified as medulloblastoma.
Among the 39 patients with cerebral shunts, 12 exhibited fevers, while 27 did not. Within the shunt infection group, 75% (9 cases) presented with fever, and 25% (3 cases) did not. Conversely, in the group without shunt infection, 11.1% (3 cases) had a fever, and 88.9% (24 cases) did not. Statistical analysis revealed a significant association between fever and shunt infection.
Examining the overall population of 39 VP shunt cases, 12 cases (30.8%) were diagnosed with shunt infection, while 27 cases (69.2%) remained infection-free. Further analysis of the infection subgroup, comprising 7 boys and 5 girls, indicated that gender did not significantly influence the incidence of VP shunt infection. Among the 12 infection cases, 41.7% (5 cases) exhibited positive CSF cultures, while 58.3% (7 cases) had negative cultures. Noteworthy pathogens identified in cases with positive cultures included Klebsiella pneumoniae (2 cases), Acinetobacter spp. (2 cases), and Enterococcus spp. (1 case).
Among the 39 patients with VP shunts, those experiencing VP shunt infection exhibited an average white blood cell (WBC) count of 546.2 in the CSF obtained from the VP shunt. In contrast, the group without VP shunt infection had an average WBC count of 1 in the CSF. An independent t-test demonstrated a statistically significant difference between the two groups concerning the WBC count in the CSF from the VP shunt (P < 0.041). Upon further examination of other variables within the files of patients with VP shunts and hydrocephalus, no significant relationships were observed between age, CSF protein levels, duration of VP shunt placement until the occurrence of VP shunt infection, and the presence of VP shunt infection (Table 2).
Abbreviation: WBC, white blood cells.
a Variables are expressed as mean ± SD.
bUsing Mann–Whitney U test.
cSignificant variable.
4. Discussion
This study investigated the epidemiology of VP shunt infections in pediatric patients with hydrocephalus, analyzing data from 39 cases admitted to Loghman Hakim Hospital between 2018 and 2023. The findings revealed that 30.8% of cases were diagnosed with VP shunt infections, with no significant gender-based differences observed. The most common etiological factor for hydrocephalus was brain tumors, particularly medulloblastoma. Fever was significantly associated with shunt infections. Additionally, the study showed a statistically significant difference in the average WBC count in CSF between the group with VP shunt infections and the group without. Other variables, including age, CSF protein levels, and the duration of VP shunt placement, showed no significant association with VP shunt infections. These findings provide valuable insights into the complex dynamics of VP shunt infections in pediatric hydrocephalic patients, highlighting the importance of specific clinical parameters in diagnosing and managing this critical condition.
A 2018 study by Erps et al., which included records of 1,570 pediatric patients aged 0 to 18 who underwent VP shunting for hydrocephalus between 1996 and 2015, reported 63 cases of VP shunt infections (15). The average time to infection occurrence was 19 days, with a higher probability of infection in children under 5 years old; notably, a history of two or more VP shunt replacements was associated with increased infection rates. In contrast, a more recent investigation of 39 pediatric cases with hydrocephalus and VP shunts at Loghman Hospital over the last 5 years found 12 cases with VP shunt infections, where the average time to infection was 216.9 days. No significant correlation was observed between age and shunt infection, and none of the infected children had a history of shunt replacement (16).
McGirt et al. conducted a 2003 study on 820 children with VP shunts for hydrocephalus, identifying VP shunt infections in 11% of cases (11). Risk factors included prematurity, prior shunt infection, and neuroendoscopy during VP shunt surgery. In the study involving 39 children with hydrocephalus and VP shunts, no history of premature birth or prior shunt infection was identified, and neuroendoscopy was not utilized, differing from the prior study (11). Moreover, a 2012 study by Lee et al. in Seoul, South Korea, involving 333 children with VP shunts, reported an average time of one month for VP shunt infection occurrence (17). However, in the present study, the average time to shunt infection was significantly longer at 216 days. Additionally, Lee et al.’s study emphasized the importance of performing VP shunt surgery before the end of the first year of life as a risk factor, which contrasts with the findings in this study (17).
In another 2012 study by Khan et al. in Karachi, Pakistan, on 113 hydrocephalic patients undergoing VP shunting, the most common causes were congenital hydrocephalus, brain tumors, and hydrocephalus following cranial surgery (18). This differed from the findings of this study, which identified brain tumors, brain structural disorders, trauma, meningitis, and myelomeningocele as primary causes.
Habibi et al.' 2016 study in Tehran on 800 VP shunt cases identified seven variables significantly associated with VP shunt infections. However, the current study, focusing on 39 children with hydrocephalus and VP shunts, did not find a significant correlation between infection and factors such as age at the time of the first VP shunt, myelomeningocele, or intraventricular hemorrhage. Additionally, there was no association between previous VP shunt infections and the current infection in this study. Notably, simultaneous infections elsewhere in the body were absent among the 12 children with VP shunt infections in this study (19).
In contrast to Skar’s exploration of CSF biomarkers for identifying CSF shunt infections, this study focused on the epidemiology of VP shunt infections in pediatric hydrocephalus patients. While Skar’s findings emphasized elevated CRP and WBC counts in shunt-infected patients, along with distinct CSF cytokine profiles between gram-positive and gram-negative infections, this research highlighted significant associations between VP shunt infections, the presence of fever, and increased WBC counts in CSF. Additionally, this study contributes to the understanding of clinical manifestations and immunological responses in pediatric VP shunt infections, aligning with Skar’s investigation into potential diagnostic biomarkers. Specifically, patients with gram-positive shunt infections displayed heightened VEGF levels, suggesting a role in CNS inflammation and blood-brain barrier disruption, warranting further exploration (20).
Furthermore, this study on the epidemiology of VP shunt infections in pediatric hydrocephalus patients contrasts with Lolansen’s systematic review, which focused on inflammatory markers in CSF from hydrocephalus patients. While Lolansen’s review identified elevated levels of various inflammatory markers, including IL-6, IL-1β, LRG, IL-18, VEGF, and IFN-γ, in CSF from hydrocephalus patients compared to control subjects, this research specifically investigated associations between clinical variables and VP shunt infections (21). Lolansen et al.’s findings suggest that these inflammatory markers may play a role in the development and progression of hydrocephalus, potentially serving as disease biomarkers and targets for pharmacological management. In contrast, this study highlighted significant associations between VP shunt infections, the presence of fever, and increased WBC counts in CSF, contributing to the understanding of clinical manifestations and immunological responses in pediatric VP shunt infections. While Lolansen et al.’s review provides insights into potential mechanisms underlying hydrocephalus pathogenesis, this study offers implications for the diagnosis and management of VP shunt infections in pediatric patients with hydrocephalus (21). It is also important to note that other microorganisms, such as Chryseobacterium gleum, can cause VP shunt infections, emphasizing the need for comprehensive microbial assessment (22).
The current study investigating the epidemiology of VP shunt infections in pediatric patients with hydrocephalus at Loghman Hakim Hospital presents several strengths. The inclusion of a relatively sizable cohort of 39 cases within a specific five-year period allows for a focused examination of the incidence and patterns of VP shunt infections in this population. The utilization of detailed medical records provides a comprehensive understanding of various clinical variables, contributing to the robustness of the findings. Moreover, the study’s emphasis on demographic characteristics, etiological factors, and clinical outcomes enhances its relevance in the context of pediatric neurosurgery and hydrocephalus management.
However, several limitations should be acknowledged. The observational nature of the study may introduce bias and limit the ability to establish causal relationships. The single-center design may impact the generalizability of the findings to broader populations. Furthermore, the absence of certain variables, such as birth weight and history of prematurity, in the available medical records limits the study’s capacity to explore potential associations. Additionally, the relatively small sample size (39 patients) compared to other studies and the lack of a dedicated pediatric neurosurgery center are other limitations. Despite these limitations, the study provides valuable insights into the specific context of VP shunt infections in a pediatric cohort, highlighting areas for further research and potential improvements in clinical practice.
4.1. Recommendations for Future Studies
It should be conducted with a larger sample of patients and across multiple centers.
4.2. Limitations
The single-center nature of the study and the small number of patients are limitations.
4.3. Conclusions
In conclusion, the study reveals a significant association between VP shunt infection and the presence of fever, as well as an increased WBC count in the CSF obtained from the VP shunt. This correlation suggests a potential immunological response triggered by the infection, where the release of cytokines may contribute to the development of fever. Concurrently, the infection stimulates an immune response, leading to the infiltration of WBCs from the peripheral blood into the CSF surrounding the VP shunt. These findings highlight the complex interplay between infection, immune response, and clinical manifestations in pediatric patients with hydrocephalus and VP shunts, offering insights into potential pathways for further exploration and targeted interventions in the management of VP shunt infections.