1. Background
Coronavirus 2019 is an emergent outbreak, officially named COVID-19. Fever, cough, fatigue, chest tightness, and difficulty breathing are common symptoms of this disease (1, 2). Real-time reverse transcription-polymerase chain reaction (RT-PCR) remains the preferred method for diagnosing COVID-19 due to its speed and accuracy in confirming active viral infection (2, 3). Severe cases are identified by respiratory distress with age-specific tachypnea, oxygen saturation of 93% or less at rest in room air, or a partial pressure of arterial oxygen to fractional inspired oxygen concentration of 300 mmHg or less. Critical cases involve respiratory failure requiring ventilation, the occurrence of shock, or multi-organ failure requiring intensive care unit management (4-6).
Given the importance of predicting disease severity for follow-up treatment of infected children and implementing timely therapeutic interventions to reduce disease risks, identifying risk factors in children is crucial. Although numerous cases in children have been reported, clinical and epidemiological patterns in this population remain less clear (7, 8).
Iran has been among the countries most affected by COVID-19, with high prevalence and mortality rates globally (9, 10). While many studies have reported clinical parameters related to COVID-19 in children worldwide, there is limited information about pediatric patients in Iran (10, 11). Identifying individuals with these risk factors can lead to preventive measures such as health recommendations and self-quarantine to mitigate the consequences of this disease.
2. Objectives
Given the different characteristics of COVID-19 in children compared to adults, there is a need for comprehensive investigations of associated factors in hospitalized children. Therefore, we aimed to assess demographic, clinical, and paraclinical findings in hospitalized children with COVID-19. Clarifying these risk factors will hopefully aid healthcare professionals in managing children affected by this disease (12, 13).
3. Methods
The present study is an analytical cross-sectional study conducted from March 2020 to March 2023 at 17 Shahrivar Hospital in Rasht, Iran. The inclusion criteria included individuals with a positive PCR test or CT scan indicative of COVID-19, confirmed by a pediatric infectious diseases subspecialist, and aged between three months and 18 years. The exclusion criteria included the incompleteness of patient file information.
Severe disease was defined by the presence of respiratory distress with tachypnea, respiratory retractions, and nasal flaring, or hypoxia (SpO2 less than 90%), or the need for intensive care unit admission, respiratory failure, septic shock, or the involvement of more than 50% of the lungs on CT scans. Patients not meeting these criteria were placed in the non-severe or mild group (14).
The sample size was determined based on Fahimzad et al.'s investigation (15), in which the frequency of heart disease in the non-severe and severe groups was 1.9% and 11.4%, respectively. Considering α = 0.05, β = 0.20, and assuming a four-fold ratio of non-severe to severe cases due to the limited number of severe cases, this resulted in a sample size of 75 individuals in the severe group and 300 individuals in the non-severe group, with a total required sample size of 375 individuals.
3.1. Data Collection and Procedure
The required data were gathered using a checklist based on the patients' medical records. It included demographic factors such as age, sex, weight, and place of residence. Clinical symptoms were also covered, including fever and its severity, shivering, body pain, sore throat, nasal discharge or congestion, anosmia, dry cough, productive cough, dizziness, sleepiness, chest pain, shortness of breath, abdominal pain, vomiting, diarrhea, and blood oxygen saturation (SpO2 above 93%, between 90 and 93%, and less than 93%).
The study also encompassed various laboratory findings, including white blood cell count (WBC) and leukocytosis, neutrophil and lymphocyte counts, and neutropenia by age according to guidelines. Additional laboratory parameters included platelet count, C-reactive protein (CRP), glucose, blood urea nitrogen (BUN), creatinine (Cr), alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), sodium, and potassium levels.
3.2. Ethical Considerations
This study was approved by the Ethics Committee of the Vice-Chancellor of Research at Guilan University of Medical Sciences (approval number: IR.GUMS.REC.1401.594).
3.3. Statistical Analysis
The obtained data were analyzed using SPSS IBM Statistics software version 21 (for Windows, version 20, USA). The normality of the quantitative data was assessed using the Kolmogorov-Smirnov test. Descriptive statistics, including mean and standard deviation, were reported for quantitative variables. For non-normally distributed data, the median and interquartile range were used. For qualitative variables, frequency and percentage were reported. The relationship between variables was examined using the chi-square test and Fisher's exact test. Additionally, independent t-tests and Mann-Whitney U tests were employed to compare quantitative variables between groups, depending on the normality of data distribution.
4. Results
In this study, 75 severe cases and 300 individuals with mild symptoms were compared. The mean age of the study participants was 7.50 ± 5.57 months, and no statistically significant difference was noted between the two groups (P-value = 0.270). Table 1 shows the demographic characteristics of the patients.
| Demographic Variables | Mild COVID-19 Cases | Severe COVID-19 Cases | P-Value |
|---|---|---|---|
| Age | 55.78 ± 46.19 | 64.84 ± 65.82 | 0.270 b |
| Weight percentile | 0.047 c | ||
| Normal | 212 (80.3) | 47 (71.2) | |
| < 5 | 16 (6.1) | 10 (15.2) | |
| > 95 | 36 (13.6) | 9 (13.6) | |
| Gender | 0.888 c | ||
| Female | 125 (42.2) | 31 (41.3) | |
| Male | 171 (57.8) | 44 (58.7) | |
| Location | 0.076 c | ||
| Rural | 111 (38.3) | 34 (50) | |
| Urban | 179 (61.7) | 34 (50) | |
| Comorbidity | 0.078 c | ||
| No | 257 (85.7) | 58 (77.3) | |
| Yes | 43 (14.3) | 17 (22.7) |
a Values are expressed as mean ± SD or No. (%).
bt-test.
c Chi-square test.
A total of 16 individuals (6.1%) in the mild disease group, compared to 10 individuals (15.2%) in the severe group, had a weight percentage below 5%, which was statistically significant (P-value = 0.047) (Table 1).
The frequency of sleepiness and shortness of breath showed a statistically significant difference between the two study groups. Sleepiness was reported in 82 cases (27.3%) in the mild group and 30 cases (40%) in the severe group (P-value = 0.032), while shortness of breath was reported in 32 cases (10.7%) in the mild group and 31 cases (13.1%) in the severe group (P-value < 0.001).
The frequencies of shivering, body pain, sore throat, nasal discharge or congestion, anosmia, dry cough, productive cough, dizziness, chest pain, abdominal pain, vomiting, and diarrhea did not show a statistically significant difference between the two study groups (P-value > 0.05).
Using the Mann-Whitney U test, the respiratory rate in the two groups was compared. The median respiratory rate in the mild cases (IQR 22-30) was 25, and in the severe cases (IQR 25-46.5) was 35, with a statistically significant difference between the two groups (P-value < 0.001). The respiratory rate in the severe group was higher than in the mild group.
In addition, heart rate was significantly different and is summarized in Table 2. Using the Mann-Whitney U test and interquartile range, the blood oxygen levels in the two groups were compared. The blood oxygen level in the severe group was significantly lower.
| Clinical Symptoms | Severity of COVID-19 | P-Value | |
|---|---|---|---|
| Mild (%) | Severe (%) | ||
| Fever | 0.553 | ||
| No | 42 (19.4) | 5 (16.1) | |
| Mild | 140 (64.8) | 23 (74.2) | |
| Severe | 34 (15.7) | 3 (9.7) | |
| Shivering | 0.176 | ||
| No | 274 (91.3) | 72 (96.0) | |
| Yes | 26 (8.7) | 3 (4.0) | |
| Body pain | 0.342 | ||
| No | 279 (93.0) | 72 (96.0) | |
| Yes | 21 (7.0) | 3 (4.0) | |
| Sore throat | 0.121 | ||
| No | 277 (92.3) | 73 (97.3) | |
| Yes | 23 (7.7) | 2 (2.7) | |
| Nasal discharge | 0.292 | ||
| No | 240 (80.0) | 64 (85.3) | |
| Yes | 60 (20.0) | 11 (14.7) | |
| Anosmia | 0.999 | ||
| No | 298 (99.3) | 75 (100.0) | |
| Yes | 2 (0.7) | 2 (0.5) | |
| Dry cough | 0.345 | ||
| No | 209 (69.7) | 48 (64.0) | |
| Yes | 91 (30.3) | 27 (36.0) | |
| Productive cough | 0.768 | ||
| No | 277 (92.3) | 70 (93.3) | |
| Yes | 23 (7.7) | 5 (6.7) | |
| Dizziness | 0.999 | ||
| No | 296 (98.7) | 74 (98.7) | |
| Yes | 4 (1.3) | 1 (1.3) | |
| Sleepiness | 0.032 | ||
| No | 218 (72.7) | 45 (60.0) | |
| Yes | 82 (27.3) | 30 (40.0) | |
| Chest pain | 0.703 | ||
| No | 294 (98.0) | 74 (98.7) | |
| Yes | 6 (2.0) | 1 (1.3) | |
| Shortness of breath | < 0.001 | ||
| No | 268 (89.3) | 44 (58.7) | |
| Yes | 32 (10.7) | 31 (41.3) | |
| Abdominal pain | 0.183 | ||
| No | 269 (89.7) | 71 (94.7) | |
| Yes | 31 (10.3) | 4 (5.3) | |
| Vomiting | 0.282 | ||
| No | 188 (62.7) | 52 (69.3) | |
| Yes | 112 (37.3) | 23 (30.7) | |
| Diarrhea | 0.364 | ||
| No | 238 (79.3) | 63 (84.0) | |
| Yes | 62 (20.7) | 12 (16.0) | |
| Respiratory rate (per minute) | 25 (22 - 30) | 35 (25 - 46.5) | 0.000 |
| Pulse rate (per minute) | 110 (100 - 120) | 120 (100 - 140) | 0.011 |
| O2 saturation (%) | 98 (97 - 99) | 92 (88 - 98) | 0.000 |
| Systolic blood pressure (mmHg) | 95 (90 - 100) | 95 (90 - 110) | 0.095 |
| Diastolic blood pressure(mmHg) | 60 (60 - 70) | 60 (60 - 68.75) | 0.71 |
Systolic and diastolic blood pressure did not show a statistically significant difference between the groups.
Using the chi-square test, the frequency of lung involvement in patients with chest X-rays showed a statistically significant difference between the two study groups (P-value < 0.05). The results are presented in Table 3.
| Imaging Results | Mild (%) | Severe (%) | P-Value |
|---|---|---|---|
| Chest X-ray | 0.013 | ||
| Yes | 2 (18.2) | 6 (85.7) | |
| No | 9 (81.8) | 1 (14.3) |
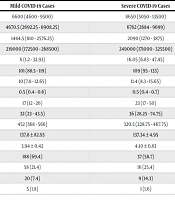
Using the Wilcoxon rank-sum test and interquartile range, the white blood cell count was compared between the mild and severe cases. The median white blood cell count in the mild cases (6600, range: 4600 - 9500) significantly differed from the severe cases (8650, range: 5050 - 13500), with a P-value of 0.003. This indicated that the severe group had a higher white blood cell count than the mild group.
The same statistical tests were applied to compare the neutrophil count between the two groups. The median neutrophil count in the mild cases (4670.5, range: 2692.25 - 6908.25) significantly differed from the severe cases (6762, range: 2684 - 9699), with a P-value of 0.027, suggesting a higher neutrophil count in the severe group compared to the mild group.
A similar analysis was conducted for lymphocyte count, platelet count, CRP, glucose, BUN, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALP, sodium, and potassium. In each case, significant differences were observed between the mild and severe groups, indicating variations in these parameters based on the severity of the disease (Table 4).
| Laboratory Variables | Mild COVID-19 Cases | Severe COVID-19 Cases | P-Value |
|---|---|---|---|
| WBC(K.µL) | 6600 (4600 - 9500) | 8650 (5050 - 13500) | 0.003 |
| Neutrophil (K.µL) | 4670.5 (2692.25 - 6908.25) | 6762 (2684 - 9699) | 0.027 |
| Lymphocyte (K.µL) | 1444.5 (810 - 2576.25) | 2090 (1270 - 3875) | 0.000 |
| Platelet (K.µL) | 219000 (172500 - 268500) | 249000 (171000 - 325500) | 0.069 |
| CRP (mg.L) | 6 (1.2 - 32.93) | 16.05 (6.83 - 47.45) | 0.005 |
| Glucose (mg.dL) | 101 (88.5 - 119) | 109 (95 - 133) | 0.029 |
| BUN (mg.dL) | 10 (7.8 - 12.65) | 11.4 (8.3 - 15.65) | 0.015 |
| Creatinine (mg.dL) | 0.5 (0.4 - 0.6) | 0.5 (0.4 - 0.7) | 0.039 |
| ALT (U.L) | 17 (12 - 26) | 23 (17 - 50) | 0.000 |
| AST (U.L) | 32 (23 - 43.5) | 36 (28.25 - 74.75) | 0.013 |
| ALP (U.L) | 452 (368 - 566) | 320.5 (228.75 - 467.75) | 0.000 |
| Sodium (meq.L) | 137.8 ± 82.93 | 137.34 ± 4.95 | 0.379 |
| Potassium (meq.L) | 3.94 ± 0.42 | 4.10 ± 0.83 | 0.104 |
Abbreviations: WBC, white blood cell; CRP, C-reactive protein; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
a Values are expressed as mean ± SD, No. (%) unless otherwise indicated.
5. Discussion
Our study presents the demographic, clinical, and paraclinical aspects of hospitalized children with confirmed COVID-19 in Iran. The results of the present study showed that among the demographic findings, children with a weight percentile below 5% were more likely to be in the severe group, and among the symptoms related to COVID-19, sleepiness and shortness of breath were significantly more common in the severe group than in the mild group. Additionally, among the clinical signs related to COVID-19, respiratory rate and heart rate were significantly higher in severe cases than in mild cases, while blood oxygen percentage was significantly lower in severe cases. In our study, lung involvement in X-rays was significantly greater in severe cases. White blood cells, neutrophils, lymphocytes, glucose, BUN, creatinine, ALT, and AST were significantly higher in severe cases than in mild cases, while alkaline phosphatase was lower in severe cases than in mild cases.
Results revealed that patients with severe COVID-19 tend to be older, although this finding is not statistically significant. In the study by Fahimzad et al., younger children were more likely to suffer from severe COVID-19 (P = 0.02) (15). The larger sample size in our study may enhance the effectiveness of the results, thereby increasing their reliability. Furthermore, the severity of the disease tends to be more pronounced in infants, while it escalates with age, particularly in adults. Although the precise pathogenic mechanisms of COVID-19 remain incompletely understood, it is evident that the disease inflicts organ damage due to the spike protein's strong affinity for the human angiotensin-converting enzyme 2 (ACE2) cell receptor, which is prominently expressed in organs such as the lungs, heart, liver, kidneys, and brain. Generally, clinical severity and mortality rates of the disease are lower in children compared to adults, potentially attributed to the reduced presence of ACE2-expressing AT2 cells and lower ACE2 protein levels in children relative to adults (16, 17).
The frequency of male gender was higher in severe cases, urging increased attention to affected boys, although more studies in this field are necessary. Studies by Hayden and Wang et al. have indicated increased mortality and morbidity associated with older age, male gender, cardiovascular diseases, diabetes, and smoking (18, 19). Williamson et al. found that older age and male gender are primary risk factors for severe outcomes in COVID-19. Many pre-existing conditions, such as cardiovascular diseases, hypertension, diabetes, respiratory diseases, and cancer, are associated with a higher risk of mortality (20).
The number of mild cases was higher among urban residents in the study. This result may be attributed to the fact that people living in cities have better access to health facilities and find it easier to reach hospitals compared to those living in rural areas.
As previously mentioned, the spike (S) protein of the virus binds to the ACE2 receptor, facilitating entry into host cells alongside the transmembrane protease TMPRSS2. In individuals whose immune response is sufficiently robust, the spread of the infection within the lower respiratory tract can be swiftly contained, resulting in either asymptomatic or mild illness. However, an inadequate initial immune response allows for unchecked viral replication, potentially leading to severe acute respiratory distress syndrome or systemic disease characterized by hyperinflammation, multiorgan failure, and prolonged recovery, often necessitating hospitalization and posing life-threatening risks. Thus, a thorough understanding of COVID-19's pathogenesis is crucial for devising effective clinical management strategies. Addressing specific pathogenic mechanisms at various stages of the disease is imperative for mitigating severity and lowering morbidity and mortality rates associated with SARS-CoV-2 infection (21).
Regarding the importance of this novel virus, we aimed to assess the symptoms of severe cases to facilitate concise predictions and rapid management. In our study, more severe cases reported fever, aligning with findings from other studies. Notably, severe cases exhibited higher rates of respiratory symptoms, such as shortness of breath. The study emphasized the correlation between the intensity of sleepiness and COVID-19 severity. In Fahimzad et al.'s study, shortness of breath was significantly higher in severe cases than in mild cases, which is consistent with our study (15). Therefore, in cases of shortness of breath, patients require more specialized care. In the study by Wang et al., fatigue and lethargy were reported more frequently in severe cases than in mild cases (P < 0.0001) (19). This aligns with our study in terms of reduced levels of consciousness; thus, if a patient is sleepy, it is advisable to provide closer monitoring.
Vital signs and laboratory parameters differed significantly between severe and mild cases. Severe cases demonstrated lower oxygen levels, higher heart and respiratory rates, and abnormal chest X-rays, which is consistent with other research. In the study by Babamahmoudi et al., high respiratory rates, elevated heart rates, and lower blood oxygen percentages during hospitalization were associated with increased mortality (7). Therefore, it is very important to assess vital signs upon hospital admission, as this can help predict disease severity (22). In the study by Sedighi et al., patchy opacities were observed in the imaging of severe cases of the disease compared to mild cases (23).
COVID-19 has the potential to trigger severe acute respiratory distress syndrome (ARDS), which may escalate to multiorgan failure. This progression is thought to be driven by the dysregulation of inflammation and the onset of cytokine storms. Given the pivotal role of inflammatory processes in severe cases, indicators such as fever, leukocytosis, and C-reactive protein levels are recognized as predictors of severe illness. Additionally, a range of other biomarkers has been identified as valuable prognostic tools for patients battling COVID-19 infection (24).
The results of this study showed that the frequencies of white blood cells, neutrophils, lymphocytes, glucose, BUN, creatinine, ALT, and AST in the two investigated groups had statistically significant differences, with all these parameters being higher in the severe group than in the mild group. Wang et al.'s study also revealed significant differences in inflammatory factors, including white blood cell count, neutrophil percentage, lymphocyte percentage, and glucose, among mild and severe groups (19). Babamahmoudi et al.'s study found an association between increased creatinine, AST, ALT, and CRP, decreased lymphocyte count, increased WBC count, and higher mortality rates (22).
5.1. Strengths and Limitations
Although we successfully assessed 375 children with COVID-19, this study had some limitations. We must acknowledge the retrospective and cross-sectional nature of the study. Examining the files makes the possibility of losing some findings inevitable, including the incompleteness of recorded examinations and requested tests in the patients' files during emergencies.
5.2. Conclusions
Our results highlighted important points regarding the demographic, clinical, and paraclinical findings in hospitalized children with COVID-19, which can shed light on this novel phenomenon. As sleepiness and shortness of breath were significantly more common in the severe group than in the mild group, the study recommended close monitoring of patients with these issues to prevent disease progression to a severe state. Given the emerging nature of COVID-19 and the importance of conducting comprehensive investigations, further prospective studies are recommended to focus on larger samples from multiple centers to validate these findings.