1. Background
Cystic fibrosis (CF) is one of the most prevalent genetic disorders among both children and adults (1). It is a multi-systemic inherited disorder caused by a primary defect in the CF transmembrane conductance regulator (CFTR) gene. In CF patients, the ability to secrete salt and water in the lungs is decreased, while the ability to reabsorb them is increased. This imbalance leads to insufficient water in the airway surface, causing secretions to become sticky, more elastic, and difficult to clear by the mucociliary system. Additionally, the pH of airway secretions becomes more acidic, further impairing mucus flow and the mucociliary clearance function (2). As a result of inadequate clearance of inhaled bacteria, these pathogens colonize the airways, triggering inflammatory responses (3).
It has also been suggested that the innate immune defense of the airways in individuals with CF is compromised, allowing colonization by microorganisms that rarely infect healthy lungs. Among these pathogens are Staphylococcus aureus, Pseudomonas aeruginosa, and Burkholderia cepacia (4-6). According to C.S. Thornton and Parkins, Staphylococcus aureus is the most common microorganism found in CF patients, and in cases of prolonged exacerbation, Pseudomonas aeruginosa may appear. Due to the high colonization rates of Staphylococcus aureus, Pseudomonas aeruginosa, and Burkholderiacepacia, these microorganisms are frequently found in CF patients (7).
In the pathogenesis of CF, it is believed that airway epithelial cells or airway surface fluids in CF patients provide an ideal environment for the growth of these microorganisms. Pseudomonas aeruginosa has been shown to have a high tendency to damage the mucosa in CF airways (8, 9). The polysaccharide structures produced by these bacteria form biofilms, creating a hypoxic environment that protects Pseudomonas aeruginosa from antimicrobial agents.
Following the initial diagnosis of CF, follow-up assessments are scheduled every 1 to 3 months, depending on the patient's age. Since the gradual and often asymptomatic decline in lung function due to infection may occur, obtaining a comprehensive history at each visit is essential (2). Indications for the use of oral antibiotics in CF patients include the presence of pulmonary symptoms and the detection of pathogenic organisms in sputum cultures. Sputum samples, either produced by the patient or collected via a swab after a severe cough, are analyzed for bacterial culture and antibiotic resistance/sensitivity. Common microorganisms that cause infection in these patients include Pseudomonas aeruginosa, Staphylococcus aureus, Burkholderiacepacia, Haemophilusinfluenzae, and other gram-negative bacilli (7). While Staphylococcus aureus can be eradicated using oral antibiotics, Pseudomonas aeruginosa remains challenging to treat (7).
Recently, the prognosis of CF has improved from being a fatal disease to a more manageable condition, with significant improvements in patients' quality of life. These improvements are attributed to earlier diagnosis, the use of appropriate antibiotics with broader microbial coverage, advanced airway clearance techniques, improved nutrition, and the availability of gene therapy (10, 11).
2. Objectives
Given the lack of sufficient research and information on the presence of unusual microorganisms in CF patients, this study aims to report the frequency of various microorganisms in the sputum cultures of CF patients. Additionally, the study evaluates the relationship between these microorganisms and the exacerbations/complications of the disease, as well as their impact on patient prognosis.
3. Methods
In this cross-sectional, descriptive-analytical study, all cases of CF patients who were referred to pediatric pulmonology clinics and/or admitted to Mofid Pediatric Hospital, Tehran, Iran, between 2017 and 2022 were included. The diagnosis of CF was confirmed based on clinical signs and symptoms and at least one positive Sweat Test (Chloride level of 60 mEq/L or more in sweat). Exclusion criteria included incomplete medical records or non-cooperation by the patient, such as refusal to attend the clinic at specific follow-up intervals or failure to respond to telephone calls. Data were collected using a paper questionnaire. Demographic and clinical history information was obtained from clinical records and/or face-to-face interviews during the patients' first visit with their parents.
The medical history of patients was recorded, including current microorganism colonization, past or current use of oral or IV antibiotics, number of exacerbations, and the need for hospitalization in the past year. Patients' prognoses were defined based on the number of exacerbations, length of hospitalization, BMI, and clinical signs and symptoms during outpatient follow-ups.
For participants aged 5 years and older, sputum samples were collected directly from the patient. For younger patients, a swab of the posterior oropharyngeal wall was used. The swabs were immediately smeared onto chocolate agar, blood agar, and MacConkey agar plates. The culture plates were incubated at 37°C for 72 hours and observed daily for colony formation. The average colony-forming units (CFUs) for each culture were reported by the same pathologist. Plates with fewer than 10 CFUs were reported as clean, following the guidelines of Blau et al. (12).
All sputum cultures that yielded negative results for bacterial growth or positive results for Candidaalbicans and/or other commensal bacteria were excluded, leaving 206 samples for analysis. Based on the review article by C.S. Thornton and Parkins on “Microbial Epidemiology of the CF Airways,” Pseudomonas aeruginosa and Staphylococcus aureus were classified as “common” microorganisms, while others were considered "uncommon" (7). Additionally, blood samples were taken from all participants to assess their 1,25 OH-vitamin D levels to evaluate vitamin D sufficiency.
3.1. Statistical Analysis
Statistical analysis was performed using IBM SPSS, version 24. The Kolmogorov–Smirnov test and Q-Q plot were used to determine the distribution pattern of quantitative variables. The chi-square test was applied to assess significant differences in qualitative variables. For quantitative variables, the One-Way ANOVA and Student's t-test were used to identify significant differences for parametric variables. A P-value of ≤ 0.05 was considered statistically significant.
3.2. Ethics Statements
The current study was registered with the Institute of Board Review at Shahid Beheshti University of Medical Sciences and was approved by the Research Ethics Committee under the reference number: IR.SBMU.MSP.REC.1399.347.
4. Results
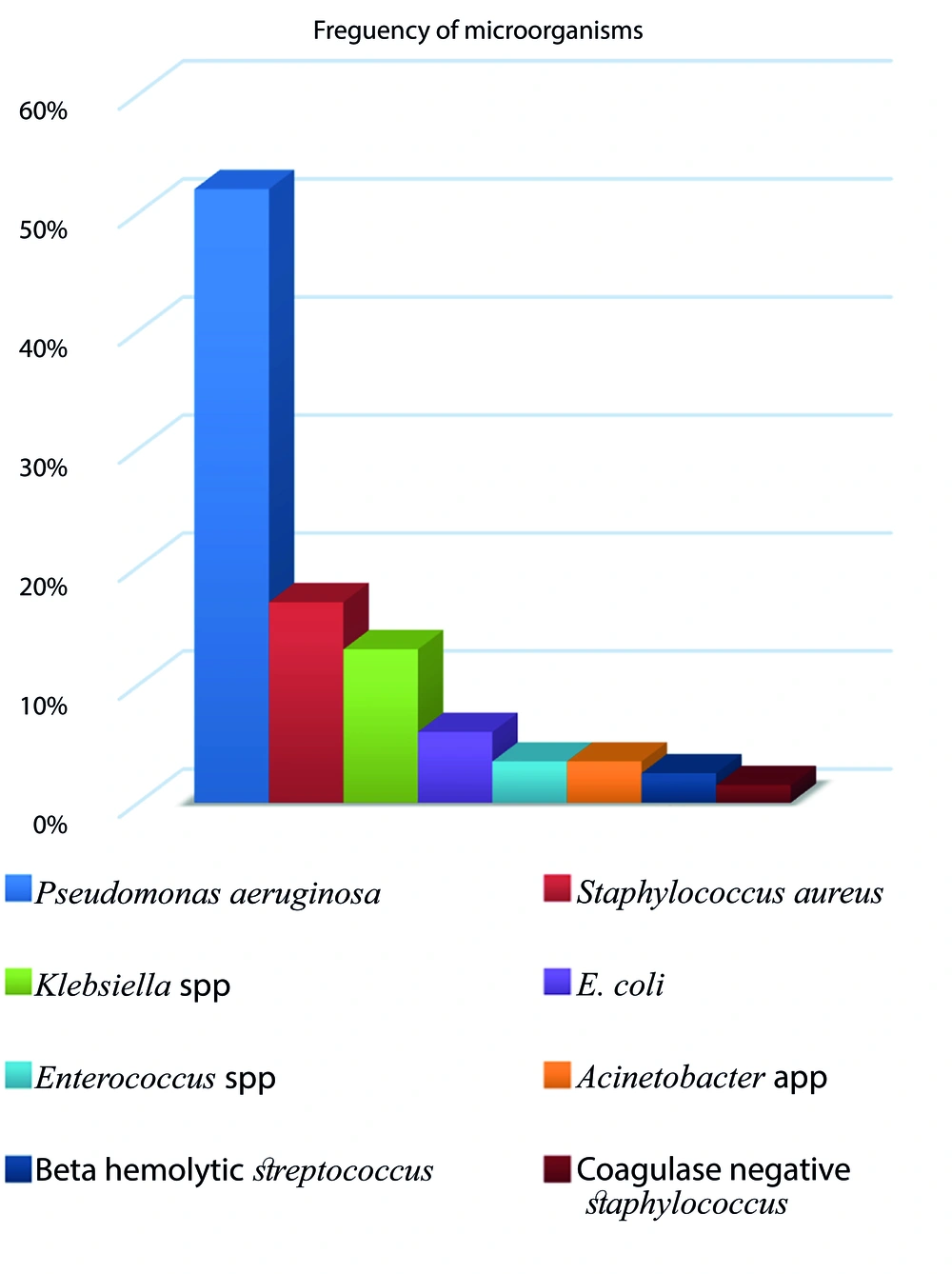
Between September 2017 and September 2022, 206 patients were included in our study (117 males and 89 females), with a mean age of 9.8 ± 5.42 years. Pseudomonas aeruginosa and Staphylococcus aureus were detected in 51.94% and 16.99% of all sputum cultures, respectively, as the most frequent microorganisms. Gram-positive Diplococcus had the lowest frequency at 0.97%. Other microorganisms identified in descending order of frequency were: Klebsiella spp. (13%), E. coli (6%), Enterococcus spp. (3.5%), Acinetobacter spp. (3.5%), beta-hemolytic Streptococcus (2.5%), and coagulase-negative Staphylococcus (1.5%) (Figure 1).
Considering SA and PA as usual organisms and the rest as unusual organisms, 68.4% of patients had sputum cultures positive for usual organisms, while the remaining cultures showed unusual organisms. Additionally, our data demonstrated that age (P-value = 0.006) had a significant statistical relationship with the type of microorganism. Older patients, with a mean age of 10.30 ± 5.77 years, were more likely to be infected with usual microorganisms, whereas unusual microorganisms were more common among younger patients, who had a mean age of 8.91 ± 4.50 years. In contrast, patients’ gender (P-value = 0.111) did not show any significant statistical relationship with the type of microorganisms.
Our results also revealed that the majority of patients had a body mass index (BMI) lower than normal, with 27% having a normal BMI, and 3% having higher than normal BMI. Pseudomonas aeruginosa and Staphylococcus aureus were more prevalent among patients with normal or lower-than-normal BMI, whereas Klebsiella spp., E. coli, Enterococcus spp., Acinetobacter spp., beta-hemolytic Streptococcus, and coagulase-negative Staphylococcus were common among those with above-normal BMI. The chi-square test, with a significance value of 0.014 in the Pearson test, indicated a significant relationship between BMI and the type of microorganisms. Furthermore, Table 1 presents the number of positive sputum cultures and the frequency of exacerbations among our cases.
| Variables | First Exacerbation | Second and More Exacerbation | Total |
|---|---|---|---|
| Pseudomonas aeruginosa | 87 (42) | 20 (10) | 107 (52) |
| Staphylococcus aureus | 32 (16) | 3 (1) | 35 (17) |
| Staphylococcus saprophyticus | 2 (< 1) | 0 (0) | 2 (< 1) |
| Coagulase negative Staphylococcus | 3 (1) | 0 (0) | 3 (1) |
| Klebsiella spp. | 23 (11) | 3 (1) | 26 (13) |
| E. coli | 10 (5) | 3 (1) | 13 (6) |
| Beta hemolytic Streptococcus | 4 (2) | 1 (< 1) | 5 (2.5) |
| Gram-positive diplococcus | 2 (< 1) | 0 (0) | 2 (< 1) |
| Enterococcus spp. | 4 (2) | 2 (< 1) | 7 (3) |
| Acinetobacter spp. | 7 (3) | 0 (0) | 7 (3) |
| Total | 174 (84.5) | 32 (15.5) | 206 (100) |
a Values are expressed as No. (%).
b The result of chi-square test depicted (P-value 0.695).
The mean age of patients with 0 or 1 exacerbation was 9.75 ± 5.46 years, while for those with 2 or more exacerbations, it was 10.50 ± 5.38 years. Additionally, the mean vitamin D level for patients with 0 or 1 exacerbation was 43.52 ± 32.41 ng/mL, whereas for those with 2 or more exacerbations, it was 40.76 ± 31.63 ng/mL. Our data did not show any significant association between the frequency of exacerbations and types of microorganisms, gender, or BMI (P-values: 0.695, 0.398, and 0.292, respectively).
Furthermore, 44.7% of patients had deficient vitamin D levels, while only 25.73% were within the sufficient range. Examination of the relationship between vitamin D levels and the type of microorganisms in sputum cultures showed no statistically significant association between these two variables (P = 0.981).
5. Discussion
In this study, we reported the frequency of various microorganisms identified in the sputum cultures of CF patients and examined the prognostic factors related to the exacerbation course in CF patients. Previous studies have suggested that Staphylococcus aureus is the most common microorganism found in the sputum cultures of CF patients (13-15). Although Pseudomonas aeruginosa has been reported as the second most common organism, a 2021 study investigating the most prevalent microorganisms in the sputum cultures of CF patients from Eastern regions suggested that Staphylococcus aureus and Pseudomonas aeruginosa were the most common microorganisms, followed by non-group A beta-hemolytic Streptococcus (16). Previous research has confirmed that Staphylococcus aureus (17) and Pseudomonas aeruginosa (18, 19)are the most frequent germs in the sputum cultures of many CF patients, thus categorizing them as "usual microorganisms" (8). Another study, however, indicated a balance between S. aureus and P. aeruginosa in CF sputum cultures (20). Several other microorganisms have also been reported as common among CF patients, including Klebsiella pneumoniae, Klebsiella oxytoca, Serratia marcescens, and Candida albicans (16).
Identifying common bacterial species in the sputum cultures of CF patients is crucial due to the recent rise in antibiotic resistance. Studies suggest that the microbiome and antibiotic resistance of CF sputum are changing over time (21). Our study also reported the presence of other microorganisms beyond the usual Staphylococcus aureus and Pseudomonas aeruginosa, including Klebsiella spp., E. coli, Enterococcus spp., Acinetobacter spp., beta-hemolytic Streptococcus, and coagulase-negative Staphylococcus, which we categorized as "unusual microorganisms" due to their rarity and low prevalence.
Additionally, Acinetobacter spp., not previously discussed in earlier studies, was identified with a prevalence of 3.40% in our sputum culture results. This microorganism was found in a two-month-old infant with CF who presented to the emergency department with apnea and was immediately intubated and transferred to the PICU, as well as in three patients aged 9, 15, and 20 years, who exhibited severe symptoms and were admitted to the PICU. All Acinetobacter spp.-positive sputum cultures were identified within the last 3 - 4 years, which may be attributed to advances in culture medium preparation and an improved understanding of this microorganism. Another possible reason for its emergence could be over-treatment with broad-spectrum antibiotics, regardless of antibiotic resistance, leading to the uncontrolled growth of Acinetobacter in sputum. Among our patients, the infant was treated with broad-spectrum antibiotics and eventually discharged, but the other three patients did not improve and died. Given the poor prognosis associated with Acinetobacter spp. in CF patients, it is crucial to raise awareness about common microorganisms and their resistance to antibiotics.
Several studies have suggested that vitamin D insufficiency and deficiency are common among CF patients (22). Patients with lower levels of vitamin D have been found to experience more frequent pulmonary exacerbations (23). Additionally, high-dose vitamin D treatment has been shown to improve outcomes in patients admitted with pulmonary exacerbations (24-26). In the present study, an evaluation of vitamin D levels in CF patients revealed that 27.64% had a vitamin D deficiency, 25.73% had sufficient levels, 15.53% had high levels, 16.50% had a severe deficiency, and 5.83% had toxic levels of vitamin D.
A comparison of vitamin D levels with the demographic characteristics of the patients showed no significant relationship between vitamin D levels and age. Instead, vitamin D levels were more closely related to the type of nutrition and the use of supplements. Patients with Pseudomonas aeruginosa-positive sputum cultures were found to have lower vitamin D levels. This microorganism is associated with higher levels of respiratory tract involvement, chronic colonization of the airways, and more frequent episodes of recurrence or exacerbation compared to other microorganisms. These exacerbations are often accompanied by symptoms such as loss of appetite, fever, and cough, which may contribute to lower vitamin D levels. Additionally, the study noted that some patients with recurrent episodes or who were hospitalized had relatively low vitamin D levels, which could be due to socioeconomic factors and insufficient nutritional intake.
Variations in vitamin D levels among CF patients during different times of the year were also linked to differences in sun exposure. CF patients should be treated with daily or weekly vitamin D supplements to maintain serum 25-OH vitamin D levels above 30 ng/mL (27, 28). Our study also revealed that only a few patients had vitamin D levels above 100 ng/mL, with no symptoms of intoxication.
Most of our patients were diagnosed with CF before the age of one. In recent years, CF has increasingly been diagnosed in infancy, particularly in patients with symptoms such as obstruction, meconium ileus, and a positive family history. Historically, CF was often diagnosed at around 6-7 years of age, but greater awareness of the disease and its prevalence in our country has contributed to earlier diagnoses, often before the age of one (9).
Finally, the majority of patients in this study had a BMI below normal, with 27% classified as normal and only 3% above normal. Our results showed a significant relationship between BMI and the type of microorganism infecting CF patients. Similar to our findings, Nagy et al. in their meta-analysis demonstrated that underweight CF patients were more prone to infection with Pseudomonas aeruginosa, though other microorganisms were not mentioned (29). Therefore, our study is novel in suggesting that BMI may influence the types of microorganisms present in CF patients. Alvarez et al. also noted that weight loss in CF patients is often linked to a lower quality of life compared to healthy individuals (30). Factors such as inadequate nutrition, treatment with multiple antibiotics that affect appetite, and the need for frequent medical visits can significantly impact a patient’s quality of life.
5.1. Limitations
One limitation of this study was the lack of patient or parental cooperation in follow-up visits, particularly in accepting the diagnosis of CF following a positive sweat test. For example, 74.8% of participants in the vitamin D measurement study were not tested due to issues such as test availability and parental refusal to cooperate. Another limitation was the COVID-19 pandemic, which began in February 2019 and led to the suspension of the spirometry clinic, preventing the evaluation of FEV1 in patients aged 6 years and older.
5.2. Conclusions
In summary, BMI and age appear to play an important role in determining the type of microorganisms infecting CF patients. Staphylococcus aureus and Pseudomonas aeruginosa were more prevalent among CF patients with normal or below-normal BMI, as well as older patients, while other microorganisms were more common among those with above-normal BMI and younger patients.