1. Background
Globally, acute respiratory diseases are the most frequent illnesses across all age groups. Typically, these diseases are confined to the upper airways and are self-limiting; however, a small percentage of cases may progress to lower respiratory tract infections (LRTIs) such as bronchiolitis and pneumonia. Pneumonia, consistently ranking among the most severe conditions causing illness and death worldwide, is characterized as an acute inflammation of the lung parenchyma and is caused by numerous microorganisms, including bacteria, viruses, and fungi (1, 2). Bacterial pathogens such as Streptococcus pneumoniae and Hemophilus influenzae, along with viral agents like respiratory syncytial virus (RSV) and influenza virus, are major contributors to pneumonia. Fortunately, vaccines are available and effective against the two bacteria and the influenza virus (3).
Clinicians treating patients with acute respiratory infections (ARI) in various hospital settings must be aware of the most common pathogens responsible for ARIs and their association with severe disease presentations to ensure appropriate patient management and treatment. Identifying the causative agents of respiratory infections is also vital for public health surveillance. However, few studies have explored the distribution of respiratory pathogens across different hospital settings (e.g., outpatient clinics, emergency rooms, inpatient wards, and intensive care units) and the environmental, etiological, and host factors that predispose patients to severe disease, including hospitalization or intensive care admission.
Some studies conducted in different geographical regions have shown that the incidence of viral infections in critical care settings varies and has often been underreported. However, over recent years, there has been an increase in reported cases, likely due to the use of more sensitive, rapid, and accurate modern diagnostic methods (4, 5).
Bacteria are the most prevalent cause of lower LRTIs, with Streptococcus pneumoniae accounting for nearly half of all documented cases of community-acquired pneumonia (CAP) where the pathogen is identified (6). However, following the pandemics of H1N1 influenza in 2009 and COVID-19 in 2019, the number of viral infections requiring intensive care unit (ICU) admission has been on the rise (7).
Prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, the prevalence of virus-associated LRTIs requiring ICU admission ranged from 16% to 49%, with RSV and influenza being the most frequently identified viruses (8, 9). Mycoplasma is a common cause of upper respiratory infections (URIs) and Lower respiratory infection (LRI), and in children, hospitalization is required in 18% of cases (10). Additionally, respiratory infections due to Chlamydia pneumoniae are significant bacterial agents in ICU settings, commonly diagnosed through serological tests. Studies show that C. pneumoniae accounts for 6% to 22% of LRI in children and adults (11).
Utilizing more advanced viral molecular diagnostic tools, it is now possible to identify etiologies that might not have been detectable in the past. This is particularly important for patients admitted to the ICU, who are more likely to have underlying medical conditions that could make them more susceptible to infections.
2. Objectives
In this study, we aimed to use accurate molecular tests to determine the rate of SARS-CoV-2 infection, as well as other respiratory viral (Influenza and RSV) and bacterial (Mycoplasma and C. pneumoniae) infections, in ICU-admitted patients during the first four waves of the SARS-CoV-2 pandemic in Iran.
3. Methods
3.1. Study Design
In this cross-sectional study, we considered 395 patients from April 1, 2020, to June 16, 2021, who were suspected or had clinically confirmed respiratory infections at Nemazi Hospital in Shiraz. The inclusion criteria were as follows: (1) being hospitalized in the ICU, (2) signing informed consent forms to participate in the study, and (3) being a resident of Fars province. The survey covered the four peaks of respiratory infections during the study period: The first peak from February to May 2020, the second from May to June 2020, the third from October to November 2020, and the fourth from March to April 2021. The full list of symptoms used to classify probable respiratory infection cases is included in the study e-questionnaire.
3.2. TaqMan Real-time PCR Assay
Sterile Dacron swabs were used to obtain nasopharyngeal fluid (NPS) samples from the patients. Respiratory specimens were collected by trained nurses regardless of the clinical severity. The collected specimens were stored on ice and transported immediately to the laboratory for viral and bacterial screening. Real-time PCR assays were conducted within 1 - 2 hours of sample collection to detect the nucleic acids of SARS-CoV-2, RSV, Influenza, Mycoplasma, and Chlamydia (12). Patient data were retrieved from the hospital information system (HIS), including demographic features such as age, sex, and clinical symptoms (fever, shortness of breath, cough, and fatigue). These data were collected via a standardized case reporting form for each enrolled patient.
3.3. Statistical Analysis
Descriptive statistics were used to classify the study group based on respiratory infection PCR results. Numbers and percentages were reported for the classified variables. There was no missing data. The chi-square test was employed to compare qualitative variables among subgroups of patients. A significance level of ≤ 0.05 was considered. To evaluate the contribution of specific viruses and bacteria without regard to the presence of other pathogens, the model included all tested respiratory viruses and their two-way interactions. All statistical analyses were performed using SPSS software, version 26.
3.4. Ethical Considerations
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (ethic code: IR.SUMS.REC.1400.268).
4. Results
4.1. Demographic Data of the Patients
A total of 395 ICU patients were enrolled in the study, comprising 209 (53.7%) male subjects. Samples were collected from hospitalized patients over a 13-month period. The mean age of the patients was (32.46 ± 27.45) years, with the youngest being 1 day old and the oldest 100 years old.
Out of the total patients, 30 (7.6%) were admitted to the COVID-19 ICU, 306 (77.5%) were admitted to non-COVID-19 ICUs, and in 59 (14.9%) cases, it was unclear which ICU the patient was admitted to. The demographic characteristics of these participants and their ICU admissions are summarized in Table 1.
| Characteristics | All Patients | COVID-ICUs | Non-COVID ICUs | Non-determined ICUs | P-Value |
|---|---|---|---|---|---|
| Number (%) | 395 | 30 (7.6) | 306 (77.4) | 59 (15) | - |
| Mean age (y) ± SD | 32.46 ± 27.45 | 50.90 ± 19.06 | 29.88 ± 27.16 | 36.43 ± 28.87 | 0.001 a, b |
| Male/female ratio | 209/180 (1.16) | 19/11 (1.72) | 163/137 (1.19) | 27/32 (0.45) | - |
| SARS-CoV-2 infections | 53 (84) | 14 (46.7) | 31 (10.13) | 8 (13.6) | < 0.0001a, c |
| Mycoplasma infections | 4 (6.25) | 0 | 4 (1.3) | 0 | 0.53 c |
| Chlamydia infections | 5 (8) | 1 (3.4) | 3 (0.98) | 1 (1.7) | 0.24 c |
| SARS-COV-2/ Mycoplasma coinfection | 1 (1.56) | 1 (3.4) | 0 | 0 | 0.001 a, c |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a P < 0.05.
b Chi-square test was employed.
c Kruskal-Wallis test was employed.
Table 1 demonstrates that the average age of patients admitted to COVID-19 ICUs was significantly higher compared to those admitted to non-COVID ICUs. While the male-to-female ratio was higher in COVID-19 ICUs, no statistically significant difference was found between the two groups.
As shown in Table 2, individuals aged 36 - 65 years constituted more than 29% of the participants in the study, making them the largest age group. They were followed by children aged ≤ 5 years, who comprised nearly 26% of the participants.
| Age (y) | Total ICU Patients | Total Mono-infection | Viral Mono- infection | Bacterial Mono- infection | Co-infection |
|---|---|---|---|---|---|
| 0 - 5 | 101 (25.56) | 4 (6.34) | 1 (2) | 3 (30) | 0 |
| 6 - 15 | 54 (13.67) | 5 (7.93) | 5 (9) | 0 | 0 |
| 16 - 35 | 68 (17.21) | 12 (19.04) | 11 (21) | 1 (10) | 0 |
| 36 - 65 | 115 (29.11) | 26 (41.26) | 23 (43) | 3 (30) | 0 |
| > 65 | 57 (14.43) | 16 (25.39) | 13 (25) | 3 (30) | 1 |
| Total | 395 | 63 | 53 | 10 | 1 |
Abbreviation: ICU, intensive care unit.
a Values are expressed as No. (%) unless otherwise indicated.
4.2. Infection Types Amongst the Intensive Care Unit-Admitted Patients
Out of the 395 patients admitted to ICUs, 63 (16%) had a single type of infection. In 53 of these cases (84%), the infection was caused by the SARS-CoV-2 virus, while 10 cases (16%) were identified as bacterial infections. Among the bacterial infections, 5 patients were diagnosed with Chlamydia and 5 with Mycoplasma. None of the ICU-admitted patients tested positive for RSV or influenza (Table 1).
Notably, one COVID-19 ICU patient aged 65 years or older was diagnosed with a dual infection of SARS-CoV-2 and Mycoplasma. Additionally, 31 ARI patients in non-COVID ICUs were found to be positive for COVID-19.
Compared to children admitted to the ICU (aged ≤ 15 years), adults (aged ≥ 15 years) were more likely to have a SARS-CoV-2 infection (19.6% vs. 3.9%), while the prevalence of bacterial infections was similar in both groups (2.9% vs. 1.9%).
4.3. Clinical Presentation of Respiratory Infections in Intensive Care Unit-Admitted Patients
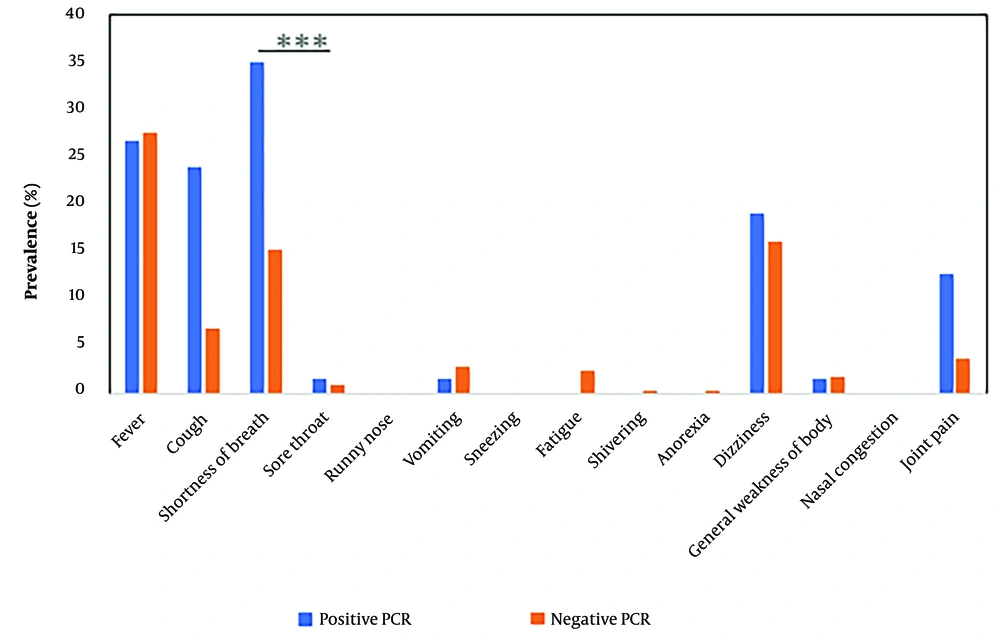
As shown in Figure 1, respiratory symptoms were compared between ICU-admitted patients with and without confirmed molecular infections. Overall, shortness of breath was the most common symptom among SARS-CoV-2-infected patients and was significantly higher (P ≤ 0.001). Additionally, coughing, joint pain, and dizziness were more prevalent among those who tested positive for infections, though these were not statistically significant.
4.4. Underlying Conditions in Intensive Care Unit-Admitted Patients
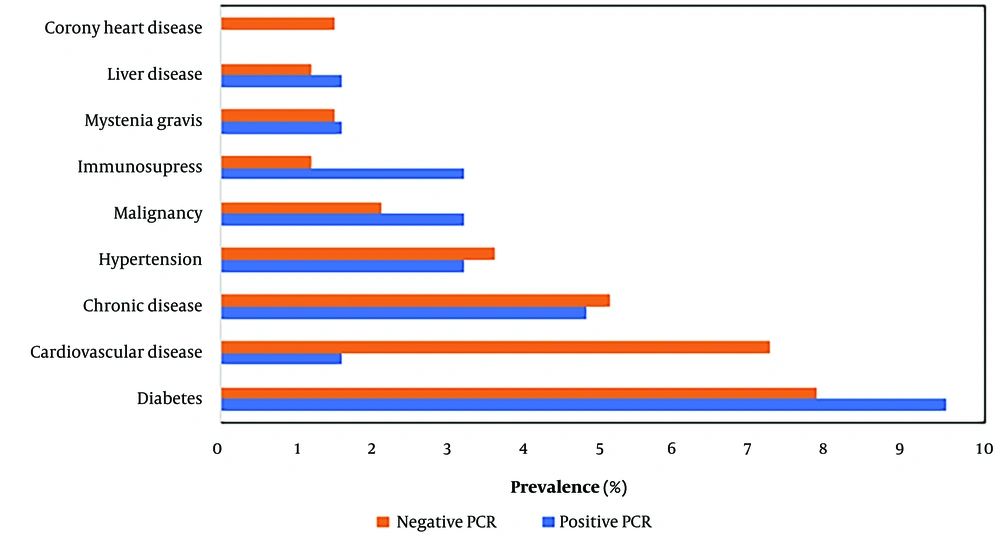
Underlying conditions were also considered in this study. A total of 146 (36.96%) patients had underlying diseases, with diabetes being the most prevalent, affecting 32 (8.1%) patients. This was followed by cardiovascular disease in 25 (6.32%) patients, chronic disease in 20 (5.06%), hypertension in 14 (3.54%), malignancies in 9 (2.27%), immunosuppressive conditions and myasthenia gravis (MG) in 6 (1.51%), liver disease in 10 (1.26%), and coronary heart disease in 10 (1.26%) patients.
Diabetes was the most frequent underlying disease among patients with confirmed respiratory infections.
Statistical analysis indicated that respiratory infections were non-significantly higher among certain groups of ICU-admitted patients with underlying conditions (Figure 2).
5. Discussion
Acute respiratory infections are one of the leading causes of mortality and morbidity worldwide, and increased attention has been given to the surveillance of ARIs among high-risk hospitalized patients following the COVID-19 pandemic. This research contributes to filling the knowledge gap regarding the extent of acute respiratory illness and provides robust data on the rate of ARIs in ICU settings. In our study, 16% of cases were classified as confirmed respiratory infections based on positive real-time PCR assays.
Severe acute respiratory syndrome coronavirus 2 was identified as the primary pathogen in ICU patients, while RSV and influenza were not detected in any of the patients. In contrast, before the COVID-19 era, viral respiratory pathogens such as influenza, RSV, and adenoviruses were the most frequently identified in patients with severe respiratory infections (13). However, by mid-2020, most countries had implemented strict infection control protocols, resulting in a significant drop in influenza and RSV cases (14-16).
Non-pharmaceutical interventions including home isolation, hand hygiene, cough etiquette, universal mask usage, and the reduction of unnecessary social activities were cited as the primary reasons for the changing patterns and declining incidence of some respiratory viral infections, including influenza and RSV, during the pandemic. Viral interference, a process that may affect viral infection patterns, has also been considered as an explanation for the decreased incidence of certain respiratory viral infections (17, 18).
The findings of this research indicated a higher rate of SARS-CoV-2 infection among adults, consistent with other studies. This could be due to decreased pre-existing immunity and increased expression and affinity of ACE-2 receptors, which facilitate viral entry (19, 20).
It is widely acknowledged that ICUs have a heightened risk of nosocomial infections, with respiratory infections being the most common. Furthermore, the risk of nosocomial respiratory infection is 20 times higher in patients requiring mechanical ventilation (21). Several studies have shown that the overall rate of bacterial infection among hospitalized COVID-19 patients was estimated to be 7.1%, with 3.5% being hospital-acquired infections and 15.5% developing nosocomial secondary bacterial infections. Despite this, 74% of patients received broad-spectrum antibiotics (22-24). In this study, the frequency of Mycoplasma and Chlamydia infections in ICU settings was relatively high (2.5%) during the pandemic. Some studies on bacterial co-infections in COVID-19 patients have indicated that Acinetobacter baumannii and Klebsiella infections are common (25-27), while this study identified Mycoplasma infections as secondary bacterial co-infections in ICU patients.
Severe outcomes among hospitalized patients with ARI were most strongly associated with the presence of pre-existing chronic diseases (28). Glezen et al. demonstrated that respiratory viruses frequently cause severe acute respiratory conditions, often resulting in hospitalization for individuals with underlying conditions (29).
Although 40% of the ICU-admitted patients in this study had underlying conditions, there was no significantly higher rate of confirmed infections among patients with or without underlying diseases.
Although it is insufficient to rely solely on clinical symptoms to identify the causative agents of respiratory tract infections (30), this study revealed that shortness of breath was the most common symptom, significantly higher in PCR-positive patients. Additionally, no significant difference in clinical symptoms was observed between confirmed cases of bacterial and viral infections in patients with ARI.
Our study had several important limitations. The sample size was limited, and all samples were baseline, without follow-up data. Lower respiratory infections encompass various diseases caused by a wide range of pathogens, which may exhibit different spatiotemporal patterns. This study assessed a restricted number of infectious agents.
5.1. Conclusions
During the COVID-19 pandemic, this study emphasized the importance of accurate identification and diagnosis of respiratory illnesses in ICUs. Our findings indicated that SARS-CoV-2 was the dominant viral infection among ICU patients, with no non-COVID viral infections such as influenza or RSV detected during the study period. However, treatable bacterial infections, including Chlamydia and Mycoplasma, were identified in ICU patients.
This research contributes to a better understanding of the ecology of viral infections during the COVID-19 pandemic and the interplay between viral and bacterial infections in ICUs.