1. Background
Viruses are the leading cause of central nervous system (CNS) infections, surpassing bacteria, parasites, and fungal agents (1). Among these, enteroviruses (EV), arboviruses, and human herpes viruses are the most common causes of viral CNS infections (2). Throughout the coronavirus 2 (SARS-CoV-2) pandemic, numerous case reports have outlined the neurological symptoms associated with the disease (3). Despite its respiratory and cardiovascular tropisms, SARS-CoV-2 is recognized as potentially neurotropic. Common neurological symptoms include loss of smell and taste, headache, muscle pain, dizziness, cognitive impairment, and disturbances of consciousness (4). Studies indicate that neurological symptoms occur in more than 35% of COVID-19 patients, with some experiencing neurological manifestations as their initial symptoms. Extensive evidence shows that SARS-CoV-2 induces modifications in cerebrospinal fluid (CSF), including elevated white blood cell counts and protein levels (5).
The CNS can be infiltrated by viruses through various mechanisms, including the olfactory nerve, retrograde transmission along cranial or peripheral nerves such as the trigeminal nerve (which has nociceptive cells in the nasal cavity), the vagus nerve, lymphatic spread, the choroid plexus, or hematogenous spread (6). Studies demonstrate that COVID-19 can penetrate the blood-brain barrier, infiltrate the CNS, and cause CNS-related symptoms directly or indirectly through circulation. However, only a few cases of SARS-CoV-2 have been detected in CSF samples (7).
Among other viral pathogens causing neurological diseases, much attention has been given to the Herpesviridae family. At least five members of this family—VZV, HSV-1, HSV-2, CMV, and EBV—are neurotropic and widespread globally (8). The primary clinical symptoms associated with these viruses include rash, fever, meningitis, behavioral changes, cognitive disability, aphasia, seizures, and neuropsychological deficits. Currently, Herpes virus neuroinvasive disease can also be diagnosed through CSF analysis, establishing correlations between clinical and laboratory findings (9, 10).
Viral neuroinvasion can result in various consequences, including acute and subacute viral encephalitis, post-infectious acute disseminated encephalomyelitis, and subacute sclerosing panencephalitis, which may occur 6-10 years after the initial viral infection (11). Human herpesviruses have also been associated with neurodegenerative diseases, including Alzheimer's disease and multiple sclerosis (12). These viruses can contribute to ischemic or hypoxic injury, cytokine storms, hyperactive inflammatory immune responses, cellular injury, strokes, and toxic metabolic alterations (13).
2. Objectives
The detection of potentially neurotropic viruses, including EBV, CMV, VZV, EVs, HSV-1 and 2, and SARS-CoV-2, in CSF samples from Namazi Hospital, a large teaching hospital in Shiraz, Iran, was performed using real-time PCR in this study. The objective was to analyze the occurrence of these viral pathogens in the CSF of patients suspected of having CNS infections during the SARS-CoV-2 pandemic.
3. Methods
3.1. Study Design
This study retrospectively examined the frequency of virus detection in CSF samples from patients with suspected acute CNS infections who were hospitalized and routinely referred to the Clinical Microbiology Research Center for suspected neurological viral infections. Assays were conducted on stored CSF samples submitted to the Clinical Microbiology Research Center of Namazi Hospital, Shiraz, Iran, from April 2021 to May 2022. During this period, the samples were kept at -70°C. Demographic, clinical, and laboratory data were collected from the electronic records of all patients. An infectious diseases specialist (Gh.R.P) reviewed published definitions for diagnosing CNS infections, including meningitis, meningoencephalitis, and encephalitis (9, 14, 15).
Meningitis was diagnosed when a patient presented with acute headache, fever, or meningism, along with a CSF leukocyte count greater than 10/high power field and, if possible, pathogen detection by either CSF PCR or blood/CSF culture (14). Patients showing meningitis signs such as altered consciousness, focal neurological symptoms, and/or abnormal EEG results were classified as having meningoencephalitis. Encephalitis was defined as altered consciousness lasting at least 24 hours without any other cause, combined with evidence of CNS inflammation and at least two of the following symptoms: Fever, seizures, focal neurological symptoms involving the brain parenchyma, CSF pleocytosis (CSF leukocyte count > 10 WBC/HPF), EEG findings suggestive of encephalitis, or neuroimaging findings suggestive of encephalitis.
The only requirement for storing a CSF sample after routine analysis was a volume of 100 μL or more. The requested molecular tests were entirely at the treating physician’s discretion and incorporated into the standard diagnostic protocol for CNS infections. For patients with multiple samples, only the first sample was included in the statistical analysis, regardless of the time interval between samples. Duplicate testing was performed on all samples. Samples from patients diagnosed with HIV infection and/or a positive CSF culture for bacteria, fungi, or mycobacteria were excluded from the analysis.
3.2. Molecular Test
Screening for SARS-CoV-2 was conducted on the selected CSF samples. A 200 µL aliquot of each sample was processed using the RIBO-prep nucleic acid extraction kit to extract viral RNA (vRNA) (AmpliSens®, Moscow, K2-9-Et-100-CE, USA) according to the manufacturer’s recommendations. Table 1 displays the primer and probe sequences for RT-qPCR, as recommended by the WHO. Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using qPCRBIO Probe 1-Step Go RT-PCR reagents (PCR Biosystems, UK), following the manufacturer’s instructions. For each reaction, 5 µL of viral RNA was combined with RT-PCR reagents, targeting the N gene, along with primers (1 µL, 10 pmol) and probe (0.5 µL, 10 pmol). Polymerase chain reaction (PCR) conditions included an initial incubation at 45°C for 15 minutes, followed by 45 cycles of 94°C for 3 minutes, 94°C for 10 seconds, and 55°C for 30 seconds. The Applied Biosystems StepOnePlus™ instrument (Thermo Fisher Scientific, Waltham, MA, USA) was used for RT-qPCR.
| Variables | Primer |
|---|---|
| SARS-CoV-2 (16) | |
| N1-F | GAC CCC AAA ATC AGC GAA AT |
| N1-R | TCT GGT TAC TGC CAG TTG AAT CTG |
| N1-P | FAM-ACC CCG CAT TAC GTT TGG TGG ACC- BHQ1 |
| N2-F | TTA CAA ACA TTG GCC GCA AA |
| N2-R | GCG CGA CAT TCC GAA GAA |
| N2-P | FAM-ACA ATT TGC CCC CAG CGC TTC AG- BHQ1 |
| HSV (17) | |
| HSV-F | CGC ATC AAG ACC ACC TCC TC |
| HSV-R | GCT CGC ACC ACG CGA |
| HSV-1P | FAM-TGG CAA CGC GGC CCA AC-BHQ1 |
| HSV-2P | JOE-CGG CGA TGC GCC CCA G-BHQ1 |
| CMV (18, 19) | |
| CMV-1F | TGGGCGAGGACAACGAA |
| CMV-1R | TGAGGCTGGGAAGCTGACAT |
| CMV-1P | FAM-TGGGCAACCACCGCACTGAGG- BHQ1 |
| CMV-2F | TCCCGCTTATCCTCRGGTACA |
| CMV-2R | TGAGCCTTTCGAGGASATGAA |
| CMV-2P | FAM-TCTCATACATGCTCTGCATAGTTAGCCCAATACA-BHQ1 |
| EBV | |
| EBV-F | CGGAAGCCCTCTGGACTTC |
| EBV-R | CCCTGTTTATCCGATGGAATG |
| EBV-P | FAM-TGTACACGCACGAGAAATGCGCC-BHQ1 |
| HHV6 | |
| HHV6-F | GTCCTCCGATCGTTGTCAGA |
| HHV6-R | TCTTAGACGTCAGGTGGCAC |
| HHV6-P | FAM-GATCACGCACATCGGTATACCTAACAG- BHQ1 |
| EV | |
| EV-F | CCCTGAATGCGGCTAATCC |
| EV-R | ARATTGTCACCATAAGCAGCCA |
| EV-P | FAM-CGGAACCGACTACTTTGGGTGTCCGTGTTTC-BHQ1 |
| JCV (20) | |
| JCV-F | TGAACCAAAAGCTACATAGGTAAGTAATG |
| JCV-R | AATCCTGTGGCAGCAG |
| JCV-P | FAM-TTCATGGGTGCCGCACTTGCA - BHQ1 |
| VZV (21) | |
| VZV-F | CGGCATGGCCCGTCTAT |
| VZV-R | TCGCGTGCTGCGGC |
| VZV-P | FAM-ATTCAGCAATGGAAACACACGACGCC-BHQ1 |
Detailed Sequence Information is Provided Below for Each Primer-Probe Combination
To identify Herpesviruses, the qPCRBIO Probe Mix Kit was used to perform one-step real-time PCR assays. For each single-reaction mixture, 5 pmol probes specific to HSV, VZV, CMV, EBV, and JC virus, along with 10 pmol primers for each virus, were combined in a total reaction volume of 20 µL. Real-time PCR was conducted on a 96-well plate using the Applied Biosystems StepOnePlus™ instrument (Thermo Fisher Scientific, Waltham, MA, USA), following these conditions: Fifty-five degrees Celsius for 10 minutes, followed by 45 cycles of 94°C for 3 minutes, 94°C for 10 seconds, and 55°C for 30 seconds. The DNA extraction and PCR procedures for the JC virus were identical to those for the Herpes family viruses. For all tests, a Ct value below 36 was considered a positive result.
3.3. Ethical Approval
Ethics Committee approval was obtained from the Shiraz University of Medical Sciences (SUMS) under the code IR.SUMS.REC.1401.468, approved on 24 November 2022.
3.4. Statistical Analysis
The current study findings were analyzed to establish a connection between the prevalence of SARS-CoV-2, VZV, HSV-1 and 2, EBV, CMV, JCV, and patients' sex and age using IBM SPSS Statistics software version 22. Differences were considered statistically significant with a P-value of less than 0.05.
4. Results
4.1. General Characteristics
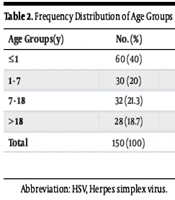
The CSF samples of 166 consecutive patients hospitalized in Nemazi Teaching Hospital with suspected meningitis were analyzed to determine the prevalence of SARS-CoV-2, HSV-1, HSV-2, CMV, EBV, EVs, VZV, HHV6, and JCV using real-time PCR. These samples were sent to the PACMRC Virology Lab for HSV infection detection. The ages of the patients ranged from newborn to 84 years. Of the 166 patients, 60 (40%) were under 1 year old, 30 (20%) were between 1 and 7 years old, 32 (21.3%) were aged 7 to 18 years, and 28 (18.7%) were older than 18 years. Additionally, 99 participants (59.3%) were male, and 63 (38.9%) were female. Seizures and sepsis were reported in 19% and 10.3% of patients, respectively. The less frequently reported clinical findings included meningitis and loss of consciousness, occurring in 9.5% and 7.8% of patients, respectively.
Table 2 presents data on the prevalence of various viral infections across different age groups, showing that the highest incidence occurred in infants aged 1 year or younger (information regarding the ages of three COVID-19 patients was unavailable). Viruses were detected in 22 (13.3%) individuals. Of the total CSF samples analyzed by PCR, 21 (12.65%) were positive for only one viral pathogen, while one (0.6%) sample was positive for two viruses (CMV and HSV-1). Among these cases, Herpes simplex virus (HSV)-1 accounted for 6.6% (11 cases), SARS-CoV-2 for 3.6% (6 cases), CMV for 1.8% (3 cases), VZV for 1.2% (2 cases), and JCV for 0.6% (1 case) of all the detected pathogens (Table 3).
| Age Groups (y) | No. (%) | HSV (Positive) | JCV (Positive) | VZV (Positive) | CMV (Positive) | COVID-19 (Positive) |
|---|---|---|---|---|---|---|
| ≤ 1 | 60 (40) | 4 | 1 | 1 | 1 | 2 |
| 1 - 7 | 30 (20) | 3 | - | - | 2 | - |
| 7 - 18 | 32 (21.3) | 2 | - | - | - | 1 |
| > 18 | 28 (18.7) | 2 | - | 1 | - | - |
| Total | 150 (100) | 11 | 1 | 2 | 3 | - |
Frequency Distribution of Age Groups
| Variables | HSV-1 Positive (11 Cases) | SARS-CoV-2 Positive (6 Cases) | CMV Positive (3 Cases) | VZV Positive (2 Cases) | JCV Positive (1 Case) |
|---|---|---|---|---|---|
| Male | 9 | 2 | 1 | 1 | 1 |
| Female | 2 | 4 | 2 | 1 | 0 |
| Fever | 8 | 0 | 2 | 0 | 0 |
| Headache | 3 | 1 | 0 | 1 | 0 |
| Nausea or vomiting | 4 | 2 | 2 | 2 | 1 |
| Rash | 0 | 0 | 1 | 0 | 1 |
| Seizure | 2 | 1 | 0 | 2 | 0 |
| Meningitis/encephalitis | 20% meningitis/80% encephalitis | 0.0 | 0.0 | 50% meningitis | 0 |
| Lymphocyte | 21.80 | 16.66 | 40.33 | 49.50 | 0.0 |
| Serum-CRP (1 - 149) (mg/dL) | 41.90 | 45 | 40.33 | 1.50 | 150 |
| CSF-WBC count (0 - 21,200) (mm3) | 65.125 | 3.33 | 3.00 | 145 | 0 |
| CSF-RBC count (0 - 85) (mm3) | 76.571 | 6.50 | 58.00 | 0 | 10 |
| CSF-glucose (10 - 80) (mg/dL) | 67.90 | 64.00 | 62.5 | 56.50 | 49 |
| CSF-protein (10 - 80) (mg/dL) | 71.00 | 33.50 | 317.5 | 94.50 | 30 |
Demographic Data and Laboratory Findings in Patients Suspected with Central Nervous System Infection
Cerebrospinal fluid analysis included cell counts, protein, glucose, and viral PCR for EBV, CMV, VZV, HSV-1 and 2, SARS-CoV-2, and JCV. Lymphocyte counts, with a 95% confidence interval (CI) of 16.68 - 8.91 and a mean of 16.67, were consistent with a viral etiology in all patients. Protein levels were within the normal range for patients with positive PCR results for JCV and SARS-CoV-2 (35.04 - 56.65). Glucose levels were also within the normal range for these patients (64.15 - 72.44 for JCV and SARS-CoV-2). These findings suggest that viral infections were not significant contributors to the clinical presentations. Further investigations are needed to clarify the role of SARS-CoV-2 in the development of meningoencephalitis, particularly in suspected CSF infections.
A brief history of four SARS-CoV-2 patients is provided below (clinical data for two patients were unavailable):
4.1.1. Case 1
On November 29, 2021, a 79-year-old woman with a history of hyperlipidemia presented to the Nemazi Hospital emergency room (ER) with confusion, abnormal focal movements, and left-hand paresis. Her symptoms had started one month before admission and had progressively worsened. The patient was afebrile and awake upon admission but disoriented to time, place, and person. Several electroencephalogram evaluations indicated secondary generalized epileptic discharge and a periodic lateralized epileptic discharge (PLED) pattern. The patient’s T2 FLAIR MRI showed gyriform foci of diffuse restriction involving the posterior aspect of the parietal lobe, suggestive of recent epilepsy postictal changes, hypoglycemia, HSV encephalitis, or Creutzfeldt-Jakob disease (CJD). Her chest CT was reported as normal.
On December 8, a lumbar puncture was performed. The CSF analysis revealed the following: Total cell count 1300 per mm³, protein 29 mg/dL, sugar 63 mg/dL, RBC count 1300 mm³, WBC count 0 mm³, segments 0, lymphocytes 0, culture no growth, and gram stain negative. The CSF analysis during the patient’s first week of admission had been reported as normal. Polymerase chain reaction tests for HSV and enterovirus in the CSF were negative. The patient was treated with anti-epileptic medication based on a clinical diagnosis of CJD. Unfortunately, her symptoms progressively worsened until she lost consciousness, with a Glasgow Coma Scale (GCS) score of 7, requiring ventilatory support within one week of admission.
During her second month of hospitalization, she developed moderate to severe pleural effusion. The patient’s CRP level increased during this time, raising suspicion of pneumonia. High-resolution computed tomography (HRCT) of the lungs and nasopharyngeal swabs for COVID-19 were performed, yielding negative results. She was treated with antibiotics (meropenem and vancomycin from January 27, 2022, to February 16, 2023) and received supportive care. Despite these efforts, she succumbed to disseminated intravascular coagulation and cardiac arrest, passing away 2.5 months after her admission.
4.1.2. Case 2
A 16-year-old male patient with a previous diagnosis of hepatoblastoma under treatment presented to our department. Five days prior to admission, he developed decreased appetite and nausea, followed by dizziness and confusion. On March 13, 2022, he was brought to the Nemazi Hospital pediatric ER due to a deterioration in his level of consciousness (LOC). His GCS score was five on admission, necessitating intubation. Unfortunately, he subsequently developed status epilepticus.
Upon presentation, he was afebrile. Physical examination revealed abdominal distension suggestive of ascites, which was later confirmed by sonography as severe free fluid in the abdominopelvic cavity. An abdominopelvic CT scan showed three liver lesions and a few mesenteric lymph nodes, indicative of malignancy. Brain imaging findings were consistent with brain edema, while CSF analysis yielded normal results.
He was eventually diagnosed with liver failure and hyperammonemia (ammonia level: 580 µmol/L) secondary to hepatocellular carcinoma. Despite medical intervention, his condition deteriorated, leading to his death approximately two weeks after hospital admission.
4.1.3. Case 3
This patient was a premature neonate (gestational age: 34 + 4), 3 days old, delivered via Cesarean section with a birth weight of 2620 gr and an APGAR score of 8 at birth. He was diagnosed with respiratory distress syndrome shortly after delivery on December 8, 2021. He was admitted to the NICU due to respiratory distress and for further evaluation to rule out the possibility of meningitis. CSF analysis results were reported as normal. During physical examination, a systolic murmur was detected, and echocardiography findings were consistent with tetralogy of fallot. The patient received antibiotics during his hospital stay and was later discharged with a diagnosis of congenital heart disease.
4.1.4. Case 4
On July 7, 2022, a 2-month-old infant was admitted to Nemazi Hospital with suspected meningitis after developing poor nutrition two days earlier. She was afebrile, and her physical examination revealed no abnormalities except for incoordination of the sucking reflex. The CSF analysis was reported as normal, and the patient was treated for sepsis with ampicillin, vancomycin, and cefotaxime. She was discharged with antibiotics two days after admission. Although all cases were diagnosed with other medical conditions, COVID-19 encephalitis could not be definitively ruled out in this patient.
5. Discussion
Viral etiologies are the leading cause of CNS infections, including meningitis and encephalitis, worldwide (8). A wide range of viral agents, including HSV, EBV, CMV, VZV, HHV6, and EV, have been reported. Additionally, COVID-19 is a multi-system disease that can affect not only the respiratory and cardiovascular systems but also the digestive and nervous systems (22). Despite numerous connections between different risk factors and CNS disorders, viral infections remain a major focus of discussion (23). Since the symptoms of these CNS diseases are non-specific, molecular analysis, such as real-time PCR, is essential to identify the causative agents. This study investigated the presence of SARS-CoV-2, Herpesviridae (HSV, CMV, VZV, and EBV), Picornaviridae (EV), and Polyomaviridae (JCV) in the CSF of patients presenting with signs of HSV meningitis and encephalitis during the COVID-19 pandemic.
In 166 CSF samples from patients with neurological symptoms tested by PCR, 22 (13.3%) were positive for one of the viruses. Among these, we detected 11, 6, 3, 2, and 1 cases of HSV-1, SARS-CoV-2, CMV, VZV, and JC, respectively, but no EBV cases were identified. This study identified 11 patients with HSV; however, detailed clinical information was available for only 8 patients, all of whom had HSV encephalitis. All patients diagnosed with HSV were treated with intravenous acyclovir and discharged without complications.
A 2017 study from Turkey reported the prevalence of HSV-1 and HSV-2 as 1.80% (24/1333) and 0.08% (1/1333), respectively (24). Another research study identified 21 samples with HSV-1 and 74 samples with HSV-2. Encephalitis was observed in most HSV-1 cases, while several HSV-2 patients exhibited symptoms of meningitis. In our previous study in 2011, we identified viruses in 46% (30/65) of patients admitted to the hospital with aseptic meningitis. Among these, 13 (43.3%) and 11 (36.7%) cases were caused by non-polio human enterovirus and mumps virus, respectively. HSV, VZV, HCMV, and HHV-6 accounted for the remaining 6 (20%) cases (25).
Two patients in our study tested positive for VZV despite the absence of any skin lesions. Other reports have documented cases of VZV CNS infection detected by PCR without the usual skin manifestations. EBV meningitis was not detected in any of the patients in the present study; however, HCMV meningitis was identified in three patients who did not have a meningitis diagnosis. Aseptic meningitis caused by HCMV and EBV is rare. According to a 2017 investigation in Turkey, a small percentage of patients were infected with other Herpesviruses, including VZV, HCMV, and HHV-6 (26). In Ukraine, among 107 patients, 12.1% had HSV-1 or HSV-2, 1.8% had VZV, 13.5% had CMV, 20.5% had EBV, 5.5% had HHV-6, 12.1% had HHV-7, and 35.5% had multiple HSV types (27).
Our study identified one patient with JCV detected in the CSF, marking the first report of JCV detection in CSF from Iran, to the best of our knowledge. JCV is a prevalent human polyomavirus that can infect individuals without causing disease. Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease that occurs when JCV is reactivated, leading to the destruction of oligodendrocytes and astrocytes (28). However, JCV meningitis and encephalitis have been reported in only a few cases involving immunocompetent patients (28, 29). Our patient was a 4-month-old boy without CSF pleocytosis who was discharged with a diagnosis of MIS-C and sepsis (30).
We reported six cases of SARS-CoV-2 infection with CSF involvement confirmed by viral RNA detection. Of these, two were a 2-day-old boy and a 2-month-old girl, both suspected of having meningitis. The CSF analysis results for these patients were normal, and they were treated for sepsis. Another case involved a 16-year-old male patient undergoing treatment for hepatoblastoma, who unfortunately died nearly two weeks after hospital admission due to HCC-associated liver failure. A 79-year-old woman with hyperlipidemia was another case; despite treatment, her condition deteriorated, and she remained on supportive care until her death 2.5 months after admission. SARS-CoV-2 RNA was retrospectively detected in the CSF samples of these patients. Although they were diagnosed with other medical conditions, COVID-19 encephalitis could not be definitively ruled out.
Unfortunately, no documentation was available for the other patients. Positive nasopharyngeal tests were not recorded for patients whose clinical data were available. Several hypotheses have been proposed to explain this neural involvement. One theory suggests that the CNS is accessed through hematogenous diffusion via a leaky blood-brain barrier or retrograde neural pathways, such as through the cribriform plate and olfactory bulb. The ability of the SARS-CoV-2 spike protein to bind to ACE-2 (angiotensin-converting enzyme 2) receptors on capillary endothelium may facilitate viral entry into the CNS.
Previous studies have indicated no correlation between a positive nasal swab sample and the presence of the virus in the CSF of neurologically symptomatic patients. To date, only a small number of patients with neurological symptoms and CSF analysis have tested positive for SARS-CoV-2 by RT-PCR in CSF. These findings suggest that viral infections were not a significant contributor to the clinical presentation. Further research is needed to clarify the role of SARS-CoV-2 in the development of meningoencephalitis, particularly in suspected CSF infections. The results section summarizes the clinical and paraclinical findings of these patients. Frequent CSF findings included occasional hyperproteinorrachia and mild lymphocytic pleocytosis.
In another study, 118 ICU patients with acute respiratory distress syndrome caused by SARS-CoV-2 infection exhibited delirium and/or abnormal neurological evaluations. An Argentinian study identified two cases of CNS involvement with positive real-time RT-PCR results in CSF. In another 2021 study, only one of 21 CSF samples tested positive for SARS-CoV-2 by RT-PCR.
A systematic review of SARS-CoV-2 patients with confirmed viral presence in CSF identified 23 cases. Seven cases (30.4%) were reported in Iran, four cases (17.4%) in Brazil, and two cases (8.7%) each in the UAE and USA. The apparent higher prevalence of SARS-CoV-2 in CSF than reported in the literature may reflect the lack of SARS-CoV-2 CSF PCR testing or non-lumbar puncture diagnostic tools. A false positive result is highly unlikely, as the PCR test for SARS-CoV-2 has near-perfect specificity.
Various factors may account for the low viral detection rate among patients suspected of CNS infections. Firstly, CSF analysis is indicated in patients presenting with a wide range of clinical manifestations caused by infectious and inflammatory diseases. A broad spectrum of infectious and inflammatory conditions may mimic CNS viral infection [Whitley et al., 1989; Adler-Shohet et al., 2003]. Additionally, the test was designed to detect nucleic acid from a limited range of viruses and other microbes potentially associated with encephalitis or meningitis. Another factor influencing the detection of viral nucleic acid is the time elapsed between the onset of neurological symptoms and CSF sampling.
The limitations of our study, conducted during the COVID-19 pandemic, significantly hindered our ability to collect a larger sample size. Furthermore, our single-center approach restricted access to CSF samples, potentially affecting the generalizability of our findings. Despite these limitations, we believe our results provide valuable insights into the viral causes of CNS infections during this period.
5.1. Conclusions
This study, conducted at Nemazi Teaching Hospital, analyzed CSF samples from patients suspected of having meningitis to identify viral pathogens. The results revealed that Herpes simplex virus-1 (HSV-1) was the most prevalent virus detected, followed by SARS-CoV-2. These findings highlight the importance of conducting viral tests in suspected meningitis cases, as accurate identification of causative agents is essential for effective diagnosis and treatment. Gaining a better understanding of the viral landscape can significantly enhance patient management and improve outcomes in meningitis cases.