1. Background
Premature rupture of membranes (PROM) refers to the tearing of the fetal membranes before the onset of uterine contractions (1). Preterm premature rupture of membranes (PPROM) occurs before 37 weeks of gestation (2). Approximately 150,000 pregnancies are affected by PPROM annually in the United States (3). The reported frequency of PPROM is 5.3% in Egypt (4), 19.2% in China (5), 9.6% in Pakistan (6), and 2.41% in Iran (7). The PPROM occurs in approximately 3% of all pregnancies and is associated with one-third of all preterm births (25% - 30%) and 18% - 20% of all neonatal deaths (8-10).
Studies have identified several maternal risk factors associated with PPROM. These include abnormal Body Mass Index (BMI) (< 18.5 kg/m2 or > 23 kg/m2), a positive history of preterm delivery or PPROM, cervical bacterial colonization and genital tract infections, chorioamnionitis, antepartum bleeding, cigarette smoking, ethnicity, gynecological surgeries, and socioeconomic status (1, 8, 11, 12).
Premature rupture of membranes adversely affects perinatal outcomes by leading to preterm delivery, oligohydramnios, intrauterine infection, placental abruption, umbilical cord compression, neonatal sepsis, intraventricular hemorrhage, bronchopulmonary dysplasia, necrotizing enterocolitis, and neurological or neuromuscular dysfunction (13-15). Although the duration of PPROM (> 12 hours) or its latency period (> 24 hours) has been identified as significant factors influencing unfavorable outcomes (16), these findings have not been confirmed by other studies (5, 17).
2. Objectives
In the present study, we assessed maternal and neonatal outcomes following PPROM at a gestational age of 20 - 36+6 weeks. The study also highlighted the prevalence of fetal and maternal deaths, fetal condition at the time of pregnancy termination, duration of neonatal and maternal hospital admission, and PPROM-related comorbidities in a tertiary maternity care hospital.
3. Methods
An observational, retrospective, cross-sectional study was conducted at Imam Khomeini Hospital, a tertiary center affiliated with Tehran University of Medical Sciences in Tehran, Iran, from March 2020 to March 2023. The study included all hospitalized pregnant women with PPROM at a gestational age of 20 to 36+6 weeks. The diagnosis of PPROM was considered the rupture of membranes before 37 weeks of gestation and was confirmed by clinical and biological diagnostic procedures (18). Patient triage involved history taking by a midwife. PPROM was diagnosed based on sonographic evidence of reduced amniotic fluid volume (19), observed vaginal watery leakage, or a positive AmniSure/fern test. To eliminate bias, suspicious cases were reviewed and consulted with the present perinatologist. Additionally, cases with false-positive tests or uncertain diagnoses were admitted and monitored for 12 to 24 hours to confirm the diagnosis.
Inclusion criteria were a definitive PPROM diagnosis between the 20th week and 36+6 weeks of gestation. Patients with a gestational age under 20 weeks or beyond 36+6 weeks were excluded. Fetal demise cases were excluded due to maternal discharge before delivery. The study also excluded patients whose medical records were inaccessible. A combination of demographic, obstetrical, and clinical data was extracted from medical records and recorded in a checklist. Data included patient admission codes, maternal age, underlying diseases, gestational age at admission, cervical length, duration of neonatal and maternal hospital admission, anthropometric values, and neonatal complications. Laboratory and radiology databases were reviewed to complete data collection. Evaluating clinical data, a body temperature above 38°C was considered as fever (20). According to laboratory findings, white blood cell (WBC) counts between 4000 and 10,000 were considered normal, below 4000 as leukopenia, and above 10,000 as leukocytosis (21). Regarding the assessment of the Amniotic Fluid Index (AFI), values above 24 cm and below 5 cm were considered polyhydramnios and oligohydramnios, respectively (22). Data related to neonatal outcomes were also gathered and recorded. The relationships between variables were analyzed to assess the impact of PPROM on neonatal and maternal outcomes. Neonatal outcomes included prematurity, respiratory distress syndrome (RDS), low Apgar score, low birth weight, surfactant administration requirement, hospital admission, and mortality. Considered maternal morbidities were fever, abnormal WBC values, or AF-related complications.
3.1. Primary and Secondary Outcomes
The primary outcome assessed was the maternal and neonatal outcomes following PPROM. The secondary outcomes included the relationships between various variables.
3.2. Ethical Consideration
This study was approved by the Ethical Committee of Tehran University of Medical Sciences in accordance with the Helsinki Declaration (IR.TUMS.IKHC.REC.1400.391). To ensure the confidentiality of patient information, data were coded instead of using patients' names. Written consent was obtained from the participants, and no additional costs were imposed on them.
3.3. Statistical Analysis
Statistical analysis was performed using the statistical package for the social sciences (SPSS) software, version 26 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD), median, minimum, and maximum for continuous variables, and as number and percentage for categorical variables. Qualitative variables were analyzed using Fisher's exact test or the χ² test. The normality of data distribution was assessed with the Kolmogorov-Smirnov test. Accordingly, non-parametric Mann-Whitney and Kruskal-Wallis tests were used for analyzing variables without a normal distribution. P-values < 0.05 were considered statistically significant.
3.4. Sample Size
Simple sampling was conducted. All hospitalized pregnant women with a diagnosis of PPROM at a gestational age of 20 to 36+6 weeks during three consecutive years (2020 - 2023) were enrolled. After initial screening, a total of 396 patients with confirmed PPROM were included in the study to evaluate maternal and neonatal outcomes.
4. Results
4.1. Descriptive Data
A total of 396 eligible pregnant women with confirmed PPROM were included in the study. The mean maternal age, BMI, and gestational age at the time of admission were 31 ± 6.29 years (min: 16; max: 49), 26.9 ± 4.36 kg/m2 (min: 15.6; max: 46), and 31.1 ± 4.8 weeks (min: 20; max: 36.6), respectively. Gestational age at the time of delivery was 20 - 26 weeks in 17.5% of cases, 26 - 34 weeks in 43.3%, and 34 - 37 weeks in 39.2%. The mean values for gravidity, parity, and abortion were 2.2 ± 1.2, 0.7 ± 0.9, and 0.4 ± 0.8, respectively. Multiple gestations were observed in 72 mothers (18.2%). The mean cervical length was 28.9 ± 10.8 mm.
Diabetes (82 cases, 20.7%), hypertensive disease (50 cases, 12.6%), and alcohol or drug consumption (3 cases, 0.8%) were the most frequent prenatal complications. The mean AFI value was 11 cm, and patients with an AFI of zero underwent spontaneous abortion or curettage surgery. Based on reported AFI values, 16.2% of pregnant women had oligohydramnios, while 13.9% had polyhydramnios.
Regarding clinical findings, fever was reported in 68 mothers (17.2%), leukocytosis was observed in 75 patients, and leukopenia in 2 cases. The most common comorbidity following PPROM was vaginal bleeding, occurring in 93 cases (39.2%), followed by labor pain leading to funneling or cervical dilation in 47 cases (20.2%). Chorioamnionitis was diagnosed in 25 cases (10.7%). Fetal distress, including low AFI or thick meconium, was identified in 23 cases (9.9%). COVID-19 was reported in 21 patients (9.0%), and other complications such as placenta previa or cord prolapse occurred in 24 cases (10.3%).
A total of 182 patients (46%) received either single or multiple doses of corticosteroids during pregnancy. The mean duration of maternal hospitalization was four days, either until delivery or until discharge with follow-up. The mode of delivery was cesarean section in 278 cases (70.2%) and normal vaginal delivery (NVD) in 44 cases (11.1%). Curettage was performed in 28 mothers (7.1%), and 46 patients (11.6%) were discharged from the hospital with a follow-up schedule or by personal consent.
Out of 396 PPROM mothers, only 153 gave birth in our center. Accordingly, data were available for 153 neonates (females: 50.5%, males: 49.5%). Among these neonates, 17 (11%) died either before birth (2 cases with gestational age 20 - 26 weeks) or after birth (15 cases with gestational age 20 - 26 weeks). Additionally, 1.3% of neonates had meconium aspiration, and 28.3% had Apgar scores < 6. Of the 153 neonates, 102 (66.7%) had RDS, but only 58 (37.9%) received surfactant. The mean duration of neonatal hospitalization was 15.6 ± 17.7 days. The mean birth weight, height, and head circumference of the neonates were 1828.8 ± 838.2 g, 43.8 ± 6 cm, and 31 ± 3.4 cm, respectively.
4.2. Analyses of Neonatal and Maternal Variables
Data analysis demonstrated that low gestational age was a significant risk factor for neonatal mortality (P = 0.002), RDS (P < 0.001), and surfactant requirement (P = 0.008). Neonates with lower birth weights were also at a higher risk of requiring surfactant (P = 0.025). However, no significant associations were found between meconium aspiration and low gestational age or birth weight (P > 0.05) (Table 1).
| Neonatal Variables | Gestational Age at Admission | Birth Weight | Body Temperature | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min-Max) | P - Value | Mean ± SD | Median (Min-Max) | P - Value | Mean ± SD | Median (Min-Max) | P-Value | |
| Meconium aspiration | 0.190 | 0.252 | 0.929 | ||||||
| Yes | 29.25 ± 0.21 | 29.25 (29.1 - 29.4) | 1355 ± 35.3 | 1355 (1330 - 1380) | 36.7 ± 0.2828 | 36.7 (36.5 - 36.9) | |||
| No | 32.14 ± 4.136 | 33.4 (20 - 36.6) | 2022.49 ± 811.73 | 2097.5 (245 - 4010) | 36.795 ± 0.6018 | 36.7 (35.6 - 40) | |||
| RDS a | 0.008 a | 0.09 | 0.303 | ||||||
| Yes | 32 ± 3.9 | 33 (20 - 36.6) | 1944.63 ± 828.5 | 1970 (245 - 4010) | 36.8 ± 0.6 | 36.7 (36 - 40) | |||
| No | 33 ± 4.2 | 34.4 (21 - 36.6) | 2152 ± 760 | 2300 (485 - 3500) | 36.7 ± 0.5 | 36.6 (35.6 - 38.2 | |||
| Surfactant use | < 0.001 a | 0.025 a | 0.605 | ||||||
| Yes | 30.5 ± 4.3 | 30.5 (20 - 36.6) | 1817 ± 882 | 1760 (245 - 3650) | 36.8 ± 0.6; | 36.8 (36 - 39) | |||
| No | 33 ± 3.7 | 34.1 (21 - 36.6) | 2136.3 ± 740 | 2128 (485 - 4010) | 36.8 ± 0.6 | 36.6 (35.6 - 40) | |||
| Neonatal birth status | 0.002 a | 0.20 | 0.896 | ||||||
| Alive | 32.5 ± 4 | 33.6 (20.2 - 36.6) | 2067.5 ± 786 | 2100 (245 - 4010) | 36.8 ± 0.6 | 36.7 (35.6 - 40) | |||
| Dead | 29.1 ± 4.1 | 29.1 (20 - 36) | 1567.8 ± 893.4 | 1265 (371 - 3650) | 36.7 ± 0.44 | 36.8 (36.2 - 37.6) | |||
Relationships Between Gestational Age at Hospital Admission, Birth Weight, and Maternal Body Temperature with Neonatal Complications
Correlations between gestational age at the time of corticosteroid administration and maternal and neonatal outcomes were also assessed. The findings indicated that the frequency of corticosteroid administration varied significantly among PPROM patients with different gestational age ranges (20 - 26, 26 - 34, and 34 - 37 weeks) (P = 0.0001), with most corticosteroid administrations occurring between 26 and 34 weeks of gestation. Corticosteroid administration at a lower gestational age was significantly associated with NVD, whereas corticosteroid therapy at a higher gestational age was significantly correlated with cesarean section (C/S) (P = 0.0001).
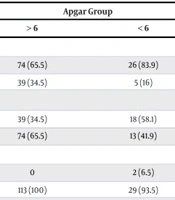
Regarding AFI groups, the results showed that oligohydramnios was more frequent among pregnant women who received corticosteroids at 20-26 weeks of gestation. In contrast, subjects who received corticosteroids after 26 weeks showed normal AFI values (P = 0.0001). Similarly, Apgar scores < 6 were more frequent among patients who received corticosteroids at a lower gestational age (P = 0.0001). Additionally, the frequency of leukocytosis was significantly higher among patients who received corticosteroids at 20 - 26 weeks of gestation compared to the other groups (P = 0.028). Table 2 provides detailed data.
| Variables | Gestational Age at the Time of Corticosteroid Administration | P-Value b | ||
|---|---|---|---|---|
| 20 - 26 (wk) | 26 - 34 (wk) | 34 - 37 (wk) | ||
| Corticosteroid use | 0.0001 c | |||
| Yes | 3 (1.6) | 105 (57.7) | 74 (40.7) | |
| No | 66 (31.1) | 66 (31.1) | 80 (37.7) | |
| Mode of delivery | 0.0001 c | |||
| NVD | 16 (36.4) | 20 (45.5) | 8 (18.2) | |
| CS | 13 (4.7) | 126 (45.5) | 138 (49.8) | |
| Amniotic fluid group | 0.0001 c | |||
| Normal | 13 (7) | 89 (47.8) | 84 (45.2) | |
| Oligohydramnios | 18 (42.9) | 16 (38.1) | 8 (19) | |
| Polyhydramnios | 4 (10.8) | 17 (45.9) | 16 (43.2) | |
| Apgar group | 0.0001 c | |||
| > 6 | 0 | 111 (44.4) | 139 (55.6) | |
| < 6 | 54 (54.5) | 38 (38.4) | 7 (7.1) | |
| White blood cells group | 0.028 c | |||
| Normal | 48 (15.1) | 137 (43.1) | 133 (41.8) | |
| Leukopenia | 0 | 2 (100) | 0 | |
| Leukocytosis | 21 (28) | 32 (43) | 22 (29.3) | |
| Fever | 0.737 | |||
| Yes | 10 (14.7) | 29 (42.6) | 29 (42.6) | |
| No | 59 (18) | 142 (43.3) | 126 (38.5) | |
Correlations Between Gestational Age Groups Based on Time of Corticosteroid Administration and Maternal/Neonatal Variables a
As shown in Table 3, there were significant relationships between Apgar scores < 6 and the occurrence of RDS (P = 0.036) as well as surfactant administration (P = 0.023). Similarly, Apgar scores < 6 were significantly correlated with meconium aspiration (P = 0.045). However, no significant relationship was found between Apgar scores and the neonatal mortality rate (P = 0.099).
Regarding the mode of delivery among PPROM patients, the results indicated that NVD was a significant factor influencing neonatal mortality (P = 0.0001). The need for surfactant administration was more frequently observed in neonates born via cesarean section (P = 0.047). However, no significant relationship was found between RDS and the mode of delivery (P = 0.242).
Further data analysis showed no significant associations between maternal fever and neonatal complications, including RDS (P = 0.518), surfactant administration (P = 0.835), meconium aspiration (P = 0.645), and neonatal death (P = 0.125). Additionally, no significant relationship was observed between maternal fever and WBC levels (P = 0.330). The prevalence of maternal fever was not clinically correlated with gestational age (P = 0.737).
Maternal WBC status was also not a statistically significant factor affecting neonatal morbidities such as RDS (P = 0.635), surfactant use (P = 0.335), meconium aspiration (P = 0.813), and neonatal mortality rate (P = 0.058). Moreover, no statistically significant relationships were found between antenatal corticosteroid administration and RDS (P = 0.494), surfactant administration (P = 0.740), meconium aspiration (P = 0.245), or neonatal mortality (P = 0.120). Additionally, no significant associations were observed between prenatal complications, such as hypertensive disorders or diabetes, and neonatal morbidities (P > 0.05).
5. Discussion
Through the present study, we aimed to understand the common outcomes, potential corresponding risk factors, and consequences of PPROM in our tertiary maternity center. Although few studies from Iran (23-25) have reported the prevalence, causes, and consequences of PPROM, our study provides more informative and comparative data.
A total of 396 patients with a gestational age of 20 - 36+6 weeks and 153 neonatal files were screened in this study to assess maternal and neonatal outcomes following PPROM. Consistent with evidence showing significant associations between prenatal complications and PPROM (11), the results of the present study identified prevalent complications such as diabetes (gestational and chronic) in 21% of cases and hypertensive disorders (gestational hypertension, preeclampsia, and chronic hypertension) in 12.6% of cases.
Diabetes can promote the production of advanced glycation end products (AGEs) and their receptors (RAGEs) (26). A recent study showed that first-trimester AGEs blood levels were significantly higher in cases complicated by PPROM (27). It was also reported that gestational diabetes mellitus could increase the risk of PPROM by 1.87-fold (11). Confirming our findings regarding the association between PPROM and hypertensive disorders, Wenas and Al-Massawi (28) reported a statistically significant relationship between gestational hypertension and PPROM. A systematic review from Iran also revealed that diabetes and maternal hypertensive disorders were among the most important risk factors for PPROM (25).
Regarding PPROM comorbidities, the results of the present study showed that vaginal bleeding was the most common event, followed by labor pain, chorioamnionitis, fetal distress, placenta previa, or cord prolapse. This finding suggests that PPROM may put the preterm fetus at risk of several complications, including vaginal bleeding, labor pain, placental abruption, ascending infection, intrapartum fetal distress, and cord prolapse.
Moreover, in contrast to existing evidence that identifies chorioamnionitis as the most significant PPROM-associated complication (29-31), our results demonstrated that this complication was not as frequent among our participants. The COVID-19 pandemic was also at its peak during the study period and significantly affected PPROM cases, with an influence rate of 5.3%.
In accordance with our findings, Gafner et al. (32) reported that vaginal bleeding, occurring in 19.7% of cases, was the most frequently observed event following PPROM among 61 included cases. The authors found chorioamnionitis and sepsis in only 8% of patients. Similarly, Addisu et al. (2) investigated obstetrical complications associated with PPROM in 424 pregnant women and reported common events such as abnormal vaginal discharge, urinary tract infections, a positive history of PPROM, and vaginal bleeding. Consistent with our results, a study by Smith et al. (8) showed a high rate of chorioamnionitis (53%) in PPROM cases with placental histopathology examination.
Regarding neonatal outcomes, the results indicated a mortality rate of 11%, Apgar scores < 6 in 28.3%, RDS in 66.7%, meconium aspiration in 1.3%, and surfactant administration in 66.7% of the 153 included neonates. Neonatal complications such as surfactant administration, RDS, and mortality were predominantly observed in neonates with lower gestational age. Moreover, surfactant use was significantly associated with low birth weight.
Consistent with our findings, an investigation from Iran demonstrated a significant relationship between PPROM and the 5th-minute Apgar score, corticosteroid administration, RDS, and other adverse neonatal outcomes (23). Kim et al. (33) reported a significant association between early-onset PPROM and neonatal mortality and morbidity. Additionally, the authors highlighted a significant relationship between PPROM and low Apgar scores in neonates with younger gestational age. Pinto et al. (34) pointed to correlations between PPROM and increased risks of RDS [odds ratio 3.05 (95% CI 1.31; 7.12)] as well as the requirement for exogenous surfactant administration [odds ratio 3.87 (95% CI 1.60; 9.35)] in 266 neonates with a gestational age of < 34 weeks. Another study by Madan et al. (35) demonstrated a significant decrease in the frequency of RDS and low Apgar scores (< 7) with advancing gestational age among 134,502 PPROM patients at 32 - 36+6 weeks of gestation. The authors also concluded that gestational age at delivery could significantly predict the risk of neonatal mortality.
Antenatal corticosteroid administration is a critical intervention to prevent neonatal morbidity and mortality. The intramuscular injection of betamethasone or dexamethasone before preterm birth reduces the risks of RDS, intraventricular hemorrhage, necrotizing enterocolitis, and death. These benefits have been observed in women with PPROM without any proven increased risk of neonatal or maternal infection (36-38).
Based on our results, the frequency of corticosteroid administration among the included participants was 46%. The highest corticosteroid administration rate was observed in the gestational age group of 26 - 34 weeks, while the lowest rate was in the 20 - 26 week group. Pauluschke‐Frohlich et al. (39) reported a lower corticosteroid administration rate of 35% in PPROM patients.
Correlations between gestational age at the time of corticosteroid administration and maternal and neonatal outcomes were also assessed. Regarding AFI groups, the results showed that oligohydramnios was more frequent among pregnant women who received corticosteroids at 20-26 weeks of gestation. Another study reported higher chances of optimal corticosteroid administration in cases diagnosed with early PPROM and oligohydramnios (39).
According to our findings, the majority of our subjects (70.2%) underwent cesarean section (CS), while 11.1% had NVD. The results also showed a relationship between corticosteroid administration at lower gestational ages and an increased frequency of NVD. Consistent with our findings, a cohort study by Bouvier et al. (11) demonstrated a potential association between PPROM and maternal outcomes in 6,968 PPROM patients, indicating a significant increase in CS as a complication of PPROM.
Contrary to our results, Jiang et al. (40) conducted a three-year investigation of 1,178 PPROM cases and found that the majority of patients underwent NVD. Similarly, a study by Sajitha et al. (41) reported that among PPROM patients with a gestational age between 28 and 38+6 weeks, 66.7% had NVD, while 31.7% underwent CS. Differences in findings across studies may be attributed to factors such as sample size and included gestational ages.
Additionally, the present study demonstrated a relationship between NVD and neonatal mortality. This finding may be related to the practice of performing vaginal deliveries in cases with a gestational age below 26 weeks to avoid the risks associated with CS. However, extremely preterm birth is inherently associated with an increased neonatal mortality rate.
Maternal leukocytosis was another variable more frequently observed among patients who received corticosteroid administration at lower gestational ages. This finding may be associated with the severity of PPROM-related complications, which necessitate early antenatal corticosteroid administration. Furthermore, the high prevalence of low Apgar scores among neonates who received corticosteroids at 20 - 26 weeks of gestation might be related to early PPROM and inevitable preterm birth.
In line with other studies (42-44), our results also demonstrated significant relationships between Apgar scores < 6 and the occurrence of RDS, meconium aspiration, and surfactant administration.
Through this study, we identified the risk factors and perinatal outcomes associated with PPROM pregnancies. Our findings highlight that this maternal complication places the fetus and neonate at risk of adverse outcomes. The gestational age at the time of PPROM emerged as the most crucial prognostic indicator for neonatal complications.
However, our study had several limitations. The primary limitation was missing data, which resulted in a relatively small sample size. Additionally, some medical records lacked precise documentation. Another constraint was the limited timeframe for conducting the study. A prospective study design, rather than a retrospective approach, might be more beneficial. Recruiting expectant mothers at high risk of developing PPROM at least a month before delivery could facilitate earlier identification of the causes of PPROM.
Several maternal and neonatal variables — such as maternal socioeconomic factors, gestational age at pregnancy termination, the cause of pregnancy termination, PPROM duration, and latency period — were not considered in this study. Finally, we recommend that before participant recruitment begins, specific criteria be established by the research faculty through an evaluation of existing guidelines. This is particularly important due to the absence of a universally recognized criterion proposed by obstetric medical communities worldwide.
5.1. Conclusions
The findings indicated that vaginal bleeding was the most common complication following PPROM. Preterm premature rupture of membranes was associated with significant perinatal mortality and morbidity. Gestational age at the time of PPROM was the most important prognostic factor for neonatal complications. These results underscore the importance of early diagnosis and timely interventions to prevent adverse outcomes. Further studies with larger sample sizes are necessary to obtain more conclusive results.